Medical device manufacturers Nvision Biomedical Technologies and Watershed Idea Foundry have received FDA clearance for what is reportedly the world’s first 3D printed titanium anterior cervical plate.
Named the Quantum Titanium Cervical Plate System, the medical device is a small plate that’s commonly used to provide stability for the spine after surgery.
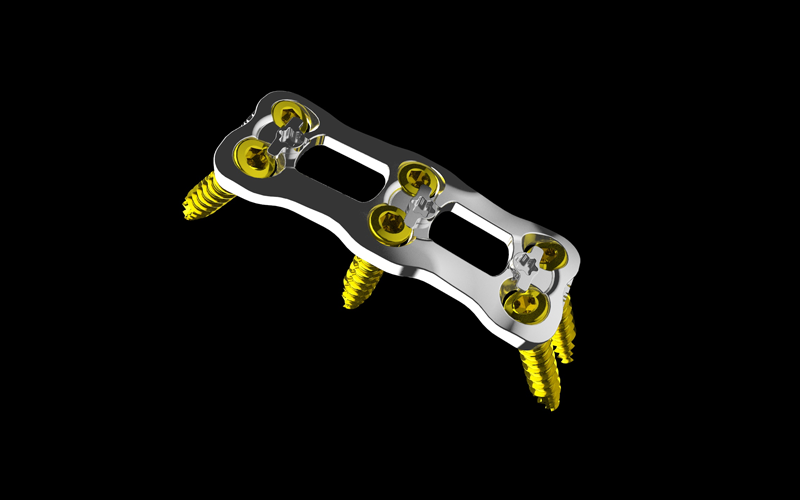
By 3D printing their cervical plate, Nvision and Watershed have been able to leverage the design benefits of additive manufacturing, combining multiple smaller components into one monolithic part. According to the firms, this greatly reduces the risk of accidental device disassembly for a patient. When compared to a traditionally manufactured counterpart, the 3D printed Quantum Titanium Cervical Plate System also lends itself to lower manufacturing costs and faster lead times.
Nvision Biomedical Technologies and Watershed Idea Foundry are sister companies operating under Fountainhead Investment Partners. They’ve previously collaborated on other 3D printed medical products such as wedges and interference screws.
“It has been incredibly exciting for us to realize new product enhancements made possible by Additive Manufacturing,” stated Nick Cordaro, CEO of Watershed Idea Foundry. “Nvision’s willingness to champion Additive Manufacturing innovation into a commercial product made for an ideal technology transfer scenario.”
What is an anterior cervical plate?
Developed back in the 1980s, anterior cervical plates are applied to the front of the spine during surgery to protect bone grafts and provide integrity to the spinal construct while it heals. They were initially used in long cervical fusions but are increasingly seeing use for anterior cervical decompression, fusion for cervical spondylosis, degenerative disc disease, and scoliosis.
Conventionally manufactured cervical plates tend to be quite costly to produce and comprise several smaller components, meaning there’s a risk they could disassemble themselves if they aren’t fitted or screwed in correctly. The Quantum Titanium Cervical Plate System is designed to address this.
Manufacturing on Demand
The Quantum Titanium Cervical Plate System
The most noteworthy innovation of the device is its monolithic nature, whereby the screw-locking cover is 3D printed as a single structure. Since there aren’t multiple components or joints involved, the plate eliminates the risk of disassembly in service.
It also features two different surface finishes, whereby the posterior surface is textured and the anterior surface is polished. The textured surface lends itself to osteointegration (bone fusion) while also absorbing some of the load that would otherwise be on the screws. Additionally, some of the screw holes have been printed at angles of 30º, a design choice that allows the plate itself to be shorter while still ensuring a robust hold on the spine.
Tom Zink, Nvision’s Senior Vice President of Product Development, explains, “By Additive Manufacturing the Quantum system, we leveraged design options that were not available with machined plates. The active locking system is integrally printed within the plates, and therefore never assembled, which means the locks cannot become unassembled in the surgical field.”

One of the healthcare sector’s most important 3D printing applications is implantable medical devices. Just recently, Health Canada, the government arm that deals with national health, approved its first Canadian-made 3D printed medical implant. The 3D printed device is a customizable mandibular (lower jaw) plate for use in facial reconstruction surgery, predominantly for patients with oral cancer. It can also be used in conjunction with surgical guides for cutting and drilling operations.
Elsewhere, 3D bioprinting firm BICO recently announced a $1.5 million contract manufacturing deal with regenerative joint replacement start-up Nanochon. The deal will see Nanochon purchase products and services worth $1.5 million from BICO’s SCIENION company to develop its 3D printed regenerative joint implants, which the start-up claims will deliver faster recoveries for patients while reducing costs to health providers.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Kubi Sertoglu

Leave A Comment