ETH Zurich-Led Study Produces Functional 3D Printed Ear Cartilage, Paving Way for Clinical Reconstruction
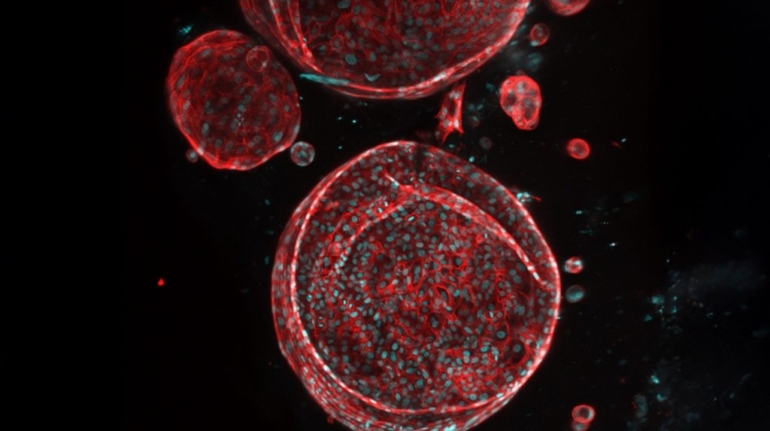
Researchers from ETH Zurich, the Friedrich Miescher Institute, and the Cantonal Hospital of Lucerne have leveraged 3D printing to engineer elastic ear cartilage with mechanical properties closely matching natural tissue. Using human ear cartilage cells, the team produced constructs that retained shape and flexibility in animal models for six weeks, marking a significant step toward patient-specific, lab-grown ear replacements.