Health Canada, the government arm that deals with national health, has approved its first Canadian-made 3D printed medical implant.
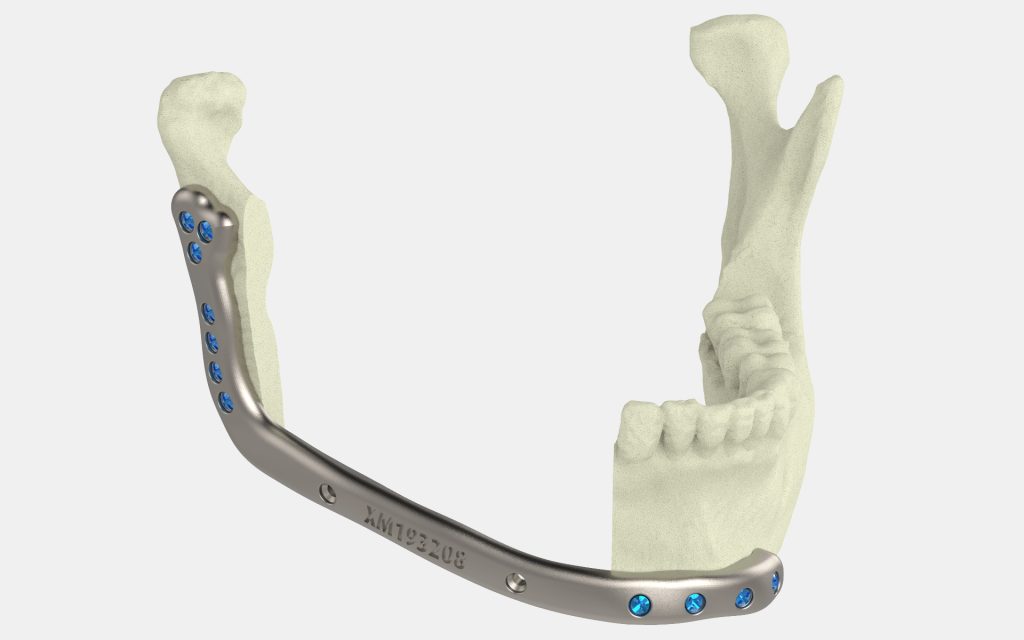
The 3D printed device is a customizable mandibular (lower jaw) plate for use in facial reconstruction surgery, predominantly for patients with oral cancer. It can also be used in conjunction with surgical guides for cutting and drilling operations.
Named the Specifit 3D mandibular plate, the implant was developed by the 3D Anatomical Construction Laboratory (LARA 3D) in Quebec City. LARA 3D is a part of Investissement Québec (CRIQ), an organization providing product development services for new enterprises. The creation of the implant was also supported by the university hospital CHU de Québec-Université Laval, orthopedic screw manufacturer Alkom Digital, and metal powder firm AP&C (a GE Additive company).
The LARA 3D laboratory was launched in late 2020 and was issued an ISO 13485 certification in April 2021, meaning it’s certified to produce new medical devices for end-use healthcare applications.
“We are extremely proud to announce Health Canada’s approval of the Specifit 3D mandibular plate,” said Dr Gaston Bernier D.M.D., Medical Chief of Dental Medicine & Oncology at CHU de Québec-Université Laval.
Oral cancer and mandibular reconstruction
For patients that have been diagnosed with oral cancer, the removal of a section of the lower jaw is sometimes a necessary procedure. In these cases, mandibular reconstruction surgery is often used to normalize the lower facial contour, regain architectural support, and improve the relationships between any affected teeth. The procedure can give patients greater functionality when it comes to both speaking and chewing, vastly improving their quality of life.
To enable mandibular reconstruction surgery, a mandibular plate is necessary. The device serves to align and stabilize the several pieces of bone that go into a reconstruction surgery, all while promoting healing and long-term bone fusion.
Bernier adds, “Not only will it improve patients’ quality of life; but thanks to optimized, guided, and personalized surgery; it will also enable the development of a 3D medical equipment center of expertise at Centre de recherche du CHU de Québec – Université Laval. We are convinced the approval of this technology marks only the start of innovation, research, and development in 3D medical printing at LARA 3D.”
Manufacturing on Demand
The Specifit 3D mandibular plate
Health Canada issued the Specifit 3D mandibular plate approval in September 2021, enabling surgeons to use the implant, together with two surgical cutting and drilling guides, to treat patients.
According to the approval, the 3D Specifit mandibular plate must be 3D printed using biocompatible materials. Medical professionals will be able to produce the implant using titanium grade 23, leveraging either laser powder bed fusion (for the implant itself) or electron beam melting (for the two surgical cutting and drilling guides).
Since the device is designed to be customizable, treatments will benefit from being patient-specific. The implant can be 3D printed according to the anatomy of the patient, which will help improve surgery success rates, as well as reduce both surgery and recovery times.
Lyne Dubois, VP of CRIQ, adds, “With LARA 3D, CRIQ fully embraces its leadership role in Additive Manufacturing in Québec. The Specifit line of 3D prostheses will meet the needs of the healthcare sector and provide improved quality of life for patients. It also demonstrates the wide range of possibilities 3D printing affords companies that must design complex, light, and durable parts. The expertise acquired by CRIQ is accessible to the entire 3D printing ecosystem in Québec.”

Just in September, Danish medical device manufacturer Particle3D was granted a Chinese patent for a novel bio-ink that enables the 3D printing of fully-resorbable porous bone implants. Composed of ceramic suspended in a fatty acid matrix, the firm’s biomaterial enables the production of patient-specific grafts with ‘bone-like’ porosity.
Elsewhere, at the University of Basel, researchers recently developed a novel 3D printed implant for treating eye socket fractures that carries a reduced risk of patient rejection. Constructed using a Prusa i3 3D printer and PEEK filament, the team’s grafts are able to overcome the bioinertness of their material thanks to their porous features, which can be tailored to enhance cellular repair.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Kubi Sertoglu

Leave A Comment