The venture capital arm of German chemicals firm Evonik has invested in Nevada-based 3D printed pharmaceutical firm Laxxon Medical to bring the mass production of 3D printed tablets closer to reality.
The firms are partnering to fabricate novel multi-drug tablets en-masse using Laxxon’s patented 3D screen printing technology and Evonik’s specialized polymer materials. The aim of the partnership is to improve drug delivery for patients while also advancing the commercialization of 3D printed pharmaceuticals.
“Drug delivery is becoming more high-precision and increasingly targets specific patient groups,” said Thomas Riermeier, Head of Evonik’s Health Care business line.
“THE COLLABORATION WITH LAXXON WILL ALLOW US TO ACCELERATE OUR ACTIVITIES IN THIS IMPORTANT AND EMERGING MARKET.”

Commercializing 3D printed pharmaceuticals
Additive manufacturing offers several advantages for clinical pharmaceutical drug development over traditional methods, such as greater personalization for individual patients and the on-demand delivery of medication. While progress is being made in the commercialization of 3D printed pharmaceuticals, challenges still remain in transitioning the technology from the lab to clinical settings.
To date, Aprecia’s 3D printed Spritam epilepsy medication is the only one to have received FDA approval, although other firms aren’t far behind. For instance, global pharmaceutical firm Merck has entered into a joint project with EOS Group company AMCM to develop and produce 3D printed tablets, initially for clinical trials and then later for commercial manufacturing.
Meanwhile, researchers at UCL, USC and FabRX have successfully used volumetric 3D printing to fabricate drug-loaded tablets in a matter of seconds, which is significantly faster than current 3D printing methods deployed for producing pharmaceuticals.

Evonik and Laxxon’s partnership
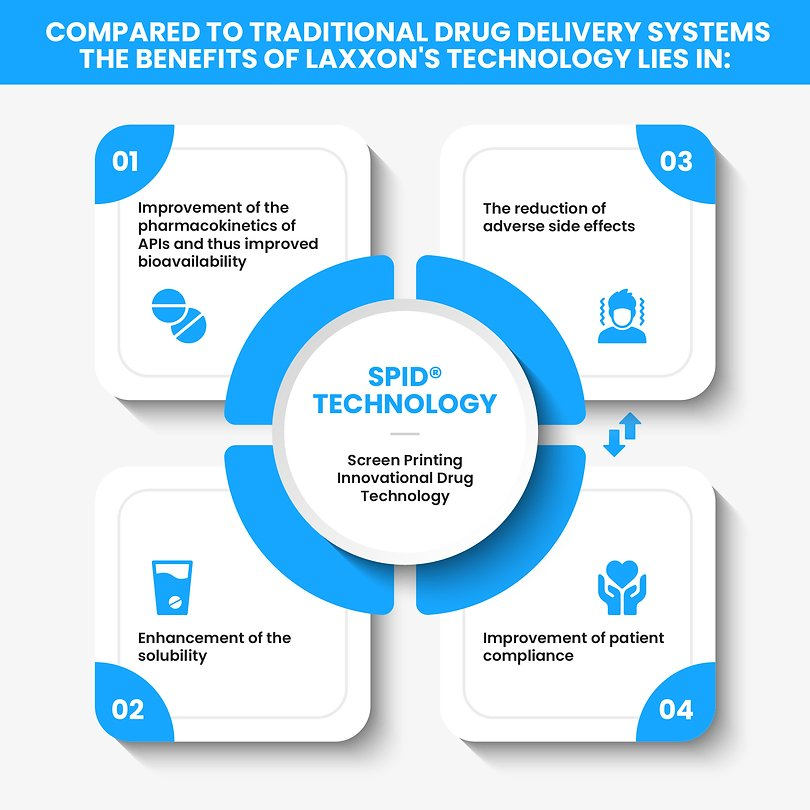
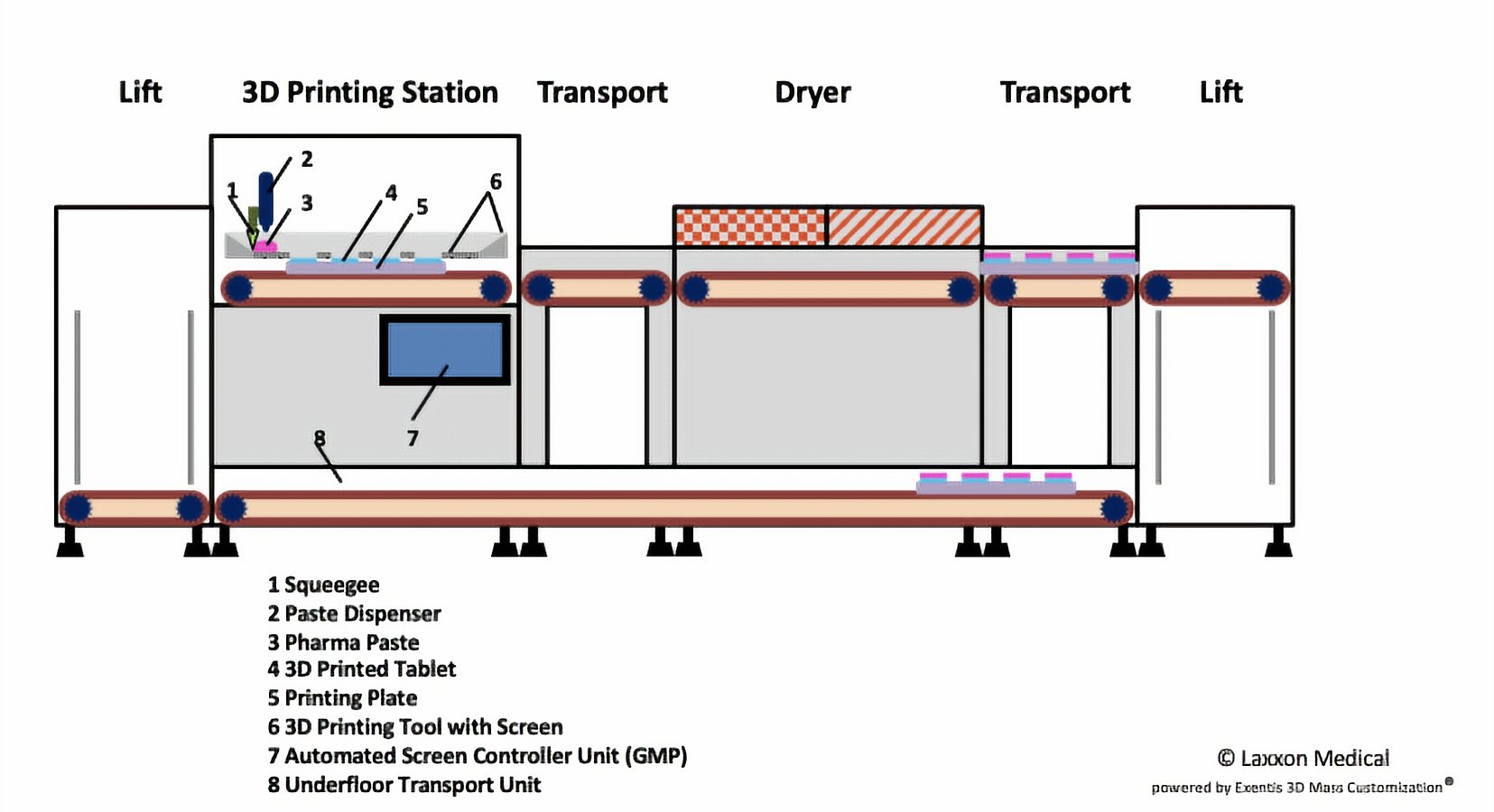
Laxxon Medical has developed a novel 3D screen printing technology specifically geared for the manufacture of structured tablets that enable the controlled release of drugs over time. The firm’s Screen Printing Innovational Drug (SPID) technology allows multiple pharmaceutical ingredients to be combined in one pill via 3D printed layers.
The 3D screen printing method enables the inner structure of the pill to contain alternative layers of active ingredients and inert layers in order to allow various doses of the drug to be released over time. The technique also permits multiple drugs to be layered on top of each other and in essence combines several pills into one.
One benefit of Laxxon’s screen printing method is its speed, which the firm claims is “significantly faster” than established 3D printing processes and could scale up the fabrication of 3D printed pharmaceuticals to mass production levels.
“This technology is great for patients,” said Bernhard Mohr, head of Evonik Venture Capital. “We expect fewer side effects from a more controlled drug delivery and having fewer pills reduces the risk of forgetting doses during the day.
“WE ARE PLEASED TO SUPPORT INNOVATION THAT BRINGS REAL BENEFITS TO PEOPLE AND THEIR HEALTH.”
With 60 years experience in drug delivery systems, Evonik makes excipients which are inactive ingredients that serve as the vehicle for a drug, such as polymers that act as a coating for tablets and allow drug release over a sustained period of time.
Through their joint product development agreement, Evonik’s products will be used in Laxxon Medical’s printing pastes to ensure the targeted delivery of the drugs within the 3D printed tablets.
The investment from Evonik’s venture capital arm will also see the firm manufacture the 3D printed tablets for Laxxon, of which the size, geometry, inner structure, and materials used combine to allow the ideal speed of chemical reactions in the body for the optimal release of drugs.
“Evonik is the perfect partner to support the development of tablets with unique release properties,” said Helmut Kerschbaumer, Co-founder of Laxxon Medical. “We are happy to have one of the world’s leading specialty chemicals companies with which we can further develop our products and at the same time commercially manufacture them.”

Leave A Comment