Regenerative medicine specialist CTIBiotech has partnered with pharmaceutical firm Gattefossé to develop 3D bioprinted skin chips that enable the patient-specific modeling of skin diseases.
By assessing a tissue’s sebum levels, the oily substance in human tissues that helps our skin barrier to function, the lab-on-a-chip devices are able to non-invasively model the skin diseases of patients. Using their chips, the firms say it could now be possible to establish a more direct link between lab data and human research, and develop more efficient cosmetic treatments.
“Bioimpedance has long been used in our bathroom scales and by dieticians to understand general body composition. Application of this to skin is a natural advance on this,” explains Prof. Colin McGuckin, President and CSO of CTIBiotech. “We advanced our 3D printed full thickness skin models with an integrated bioimpedance chip connected to monitor changes. Linking cosmetics screening in this way advances faster towards human tests.”
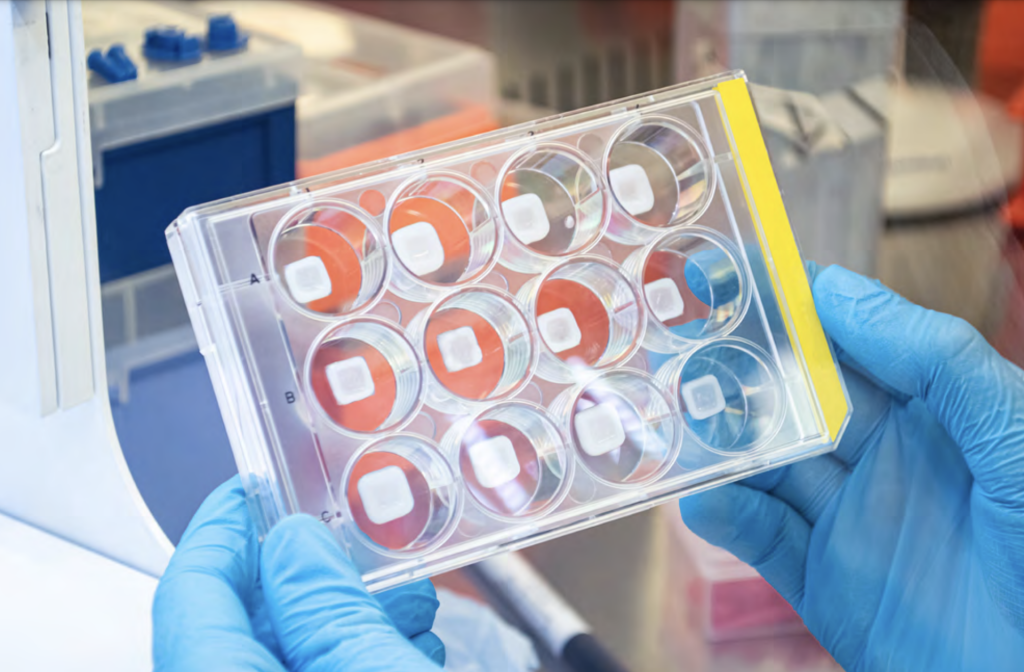 Gattefossé and CTIBiotech’s 3D bioprinted skin diagnostic platform. Photo via CTIBiotech.
Gattefossé and CTIBiotech’s 3D bioprinted skin diagnostic platform. Photo via CTIBiotech.
CTIBiotech’s bioprinting technology
Based in the commune of Meyzieu, CTIBiotech specializes in developing advanced drug screening tools. Traditionally, the firm has focused on 3D bioprinting cancer models that can be used to determine the most effective treatment for specific patients, but it has also expanded into tissue diagnostics in recent years.
In the past, CTIBiotech has worked with CELLINK to investigate new 3D bioprinted cancer therapies. Using lab-developed tumor models, the pair aimed to improve the 40% attrition rate associated with pre-clinical drug screenings.
More recently, in November 2021, the company managed to develop 3D bioprinted colon cancer models alongside the Medical University of Plovdiv and Bulgaria’s UMHAT-Eurohospital. Together, the organizations came up with a platform capable of producing cost-effective and reproducible human colon cancer disease models, with chemotherapeutic screening potential.
On the skincare front, CTIBiotech has also partnered with BASF’s Care Creations arm to conduct 3D bioprinted skin gland research. Unveiled at the NYSCC Cosmetic Congress, the initial results of the firms’ research demonstrated the successful ex-vivo production regulation of sebum, a waxy, protective matter secreted by the sebaceous glands in most skin cells.
Targeting patient-specific skin modeling
Sebum is essentially a complex mixture of lipids, the multifunctional molecules that form the building blocks of the human body. When it comes to maintaining our skin barrier, the molecules secreted by sebocytes and deposited in the stratum corneum (the outermost layer of the epidermis), are considered critical. As such, disrupting sebum production is heavily linked to the development of acne or atopic dermatitis, as well as oily or dry skin conditions.
However, while scientists understand the molecule’s role in the body, they haven’t yet managed to draw a straight line between sebum disruption and skin disease in specific patients. To rectify this, CTIBiotech and Gattefossé say more predictive testing is required that links lab testing to humans, as the former “often fails because of no proper translational readouts.”
Manufacturing on Demand
Working together, the firms have sought to establish this lab-human data link through the creation of a 3D bioprinted model based around ‘bioimpedance.’ Widely used as a measure of health, body composition and diet, the analysis method sees a current applied to patients, to calculate impedance (resistance), and assess if they need to make lifestyle changes accordingly.
By applying the same principle to assess changes in electrical activity within a 3D skin model, Gattefossé and CTIBiotech have now unlocked the ability to monitor sebum production in real-time. According to Gattefossé Research Manager Dr. Nicolas Bechetoille, the resulting lab readouts around cellular, matrix and tissue development makes their model a unique non-invasive diagnostic tool.
“Full thickness skin models containing sebocytes have reproducible oil production, and remarkably this is characterized by significant changes in bioimpedance,” adds Bechetoille. “Bioimpedance, linked to the sebum production, thus proves to be an in-vitro non-invasive proper parameter and measurable in real-time, to design ever more predictive and effective testing.
“3D models described here and linked with a simple chip system, accurately mirror changes within skin models as on live donors.”

Treating skin disorders with 3D printing
CTIBiotech is now one of many 3D bioprinting firms and research groups seeking to use the technology to advance the development of skin disease diagnostic tools. As long ago as 2020, T&R Biofab and HK inno.N unveiled plans to test new autoimmune and skin disorder drugs on a 3D bioprinted human skin model.
Elsewhere in Korea, Pohang University of Science and Technology (POSTECH) and Pusan National University have also developed 3D printed diabetic skin disease models. Leveraging the tissues, the companies aimed to establish the efficacy of various diabetes medications, and create a future replacement for animal testing in the medical and cosmetics industries.
You might also like:
Innovative 3D printing-enabled strain sensor unlocks real-time monitoring of cancerous tumors: Composed of a 50-nm layer of gold, drop-casted onto a flexible polymer enclosed within a 3D printed housing, the device is designed to be wrapped directly around tumors. Once situated, the team’s stretchable sensor expands and shrinks as tumors progress while gathering data, in a way that could provide doctors with a low-cost, scalable new way of assessing how patients respond to treatment.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment