Pharmaceutical 3D printing specialist FabRX, alongside researchers from University College London (UCL) and Spain’s Universidade de Santiago de Compostela (USC), have 3D printed a novel drug-loaded medical device to treat dry eye disorder.
The team used a digital light processing (DLP) 3D printing method to produce punctal plugs, which are small medical devices inserted into the tear duct of an eye to block it and prevent liquid drainage.
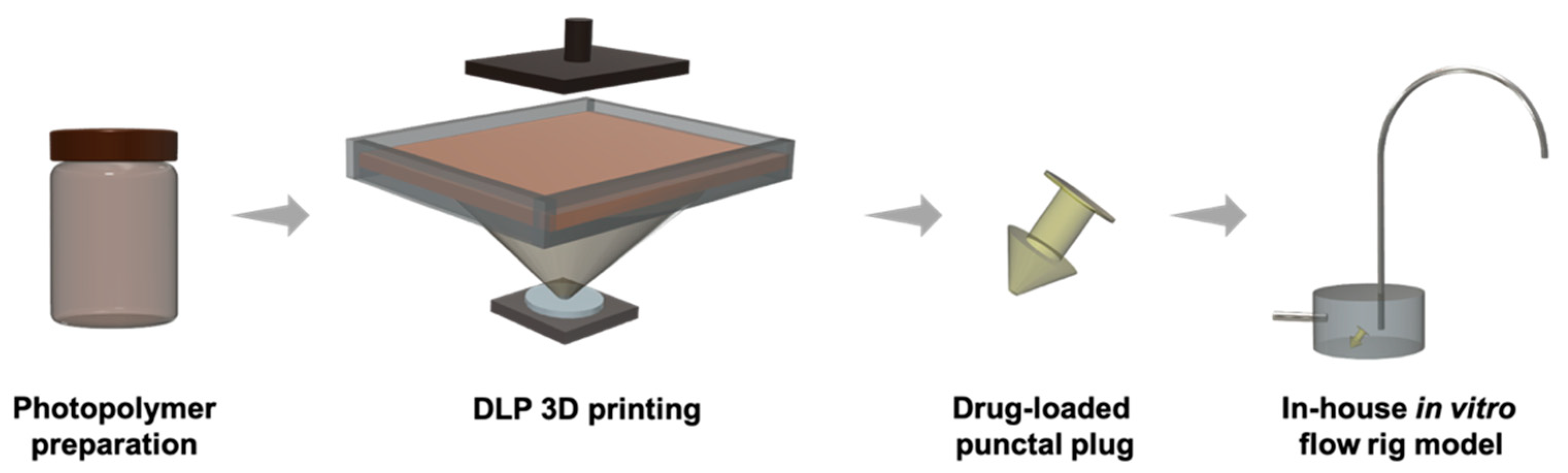
Loaded with dexamethasone, an anti-inflammatory medication, the 3D printed punctal plugs are designed to mitigate dry eye symptoms and provide an alternative treatment method to topical administration, such as eye drops.
FabRx’s 3D printed medicines
FabRx is UCL spin-out that has been leveraging 3D printing technologies to create personalized medicines and drug delivery devices for some time. Although the technology is largely in the developmental phase, 3D printing offers several significant advantages for clinical pharmaceutical drug development, such as the ability to personalize medicines to individual patient needs, expedite drug delivery timelines, and provide on-demand medication to those in hospitals, pharmacies and hard-to-reach areas.
FabRx has worked with UCL and USC in this area before, having developed personalized medicine for children with the rare metabolic disorder maple syrup urine disease (MSUD). In 2019, the partners fabricated 3D printed chewable tablets, called Printlets, which offered similar effectiveness but higher patient acceptability rate compared to conventional treatments for the disease.
Shortly afterward, FabRX debuted its M3DIMAKER 3D printer at ILMAC Exhibition in Switzerland. The desktop-sized FFF system is specially designed to enable the manufacture of personalized drug delivery devices in the required doses selected by clinicians.
Last year, FabRx used an SLS 3D printing process to produce the Printlets with Braille and Moon patterns on the surface in order to aid medicine taking for patients with visual impairment, and most recently published a paper evaluating both the state-of-play of 3D printed pharmaceuticals and the clinical potential of the technology.
Combating dry eye
For their latest partnership, FabRx, UCL and USC have explored how 3D printed medical devices could provide an alternative treatment method for dry eye, a common chronic disorder that affects millions of people worldwide. Dry eye occurs as a result of deficient tear production or increased evaporation of the tear film, and can lead to inflammation of the cornea and infections such as conjunctivitis if left untreated.
Dry eye syndrome is typically treated via topical administration methods, such as eye drops, however according to FabRx ocular bioavailability – the proportion of a drug that enters the circulation when introduced into the body – from such formulations tends to be poor.
The use of punctal plugs is a non-invasive treatment strategy to mitigate dry eye syndrome, and works by blocking the tear duct to prevent tear drainage and improve tear film stability. Photopolymerization-based 3D printing techniques such as DLP offer excellent feature resolution and a smooth surface finish, and because they operate at room temperature can also avoid thermal degradation of drugs within medical devices.
Manufacturing on Demand

DLP 3D printing would also allow for the preparation of various sizes, shapes, and drug doses of punctal plugs in a single step, and as such the partners sought to assess their capabilities over conventionally manufactured punctal plugs and topical administration methods.
3D printing the punctal plugs
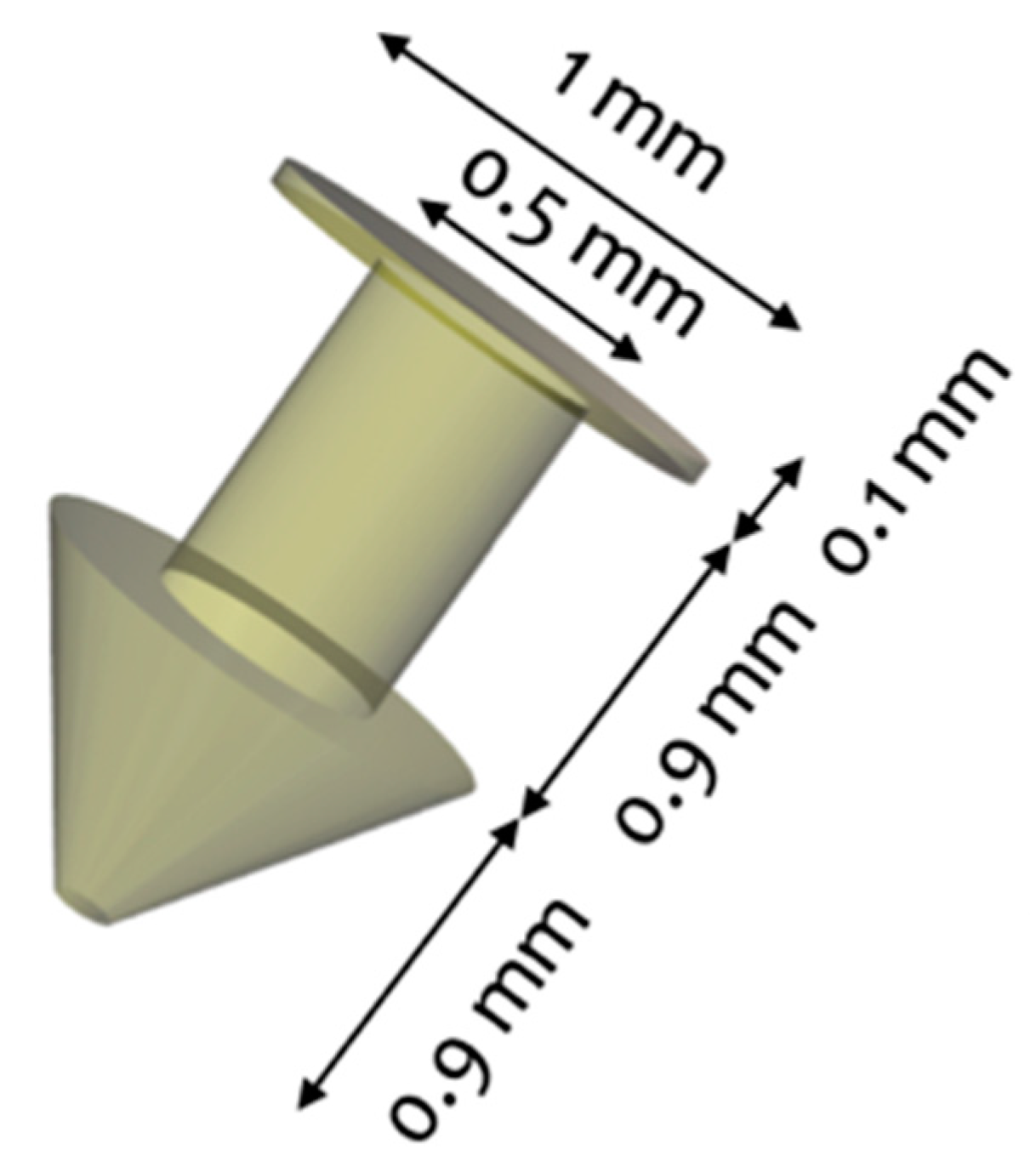
During the study, different formulations of drug-loaded punctal plugs were prepared in polyethylene glycol diacrylate (PEGDA) hydrogels via direct DLP 3D printing. The punctal plugs were designed to be inserted into the punctum of the eye with a configuration similar to that of commercially available punctal plugs, ranging from 1-2 mm in length and 0.2-1 mm in diameter.
The punctal plugs were successfully printed using a commercial DLP 3D printer, namely the Titan2 HR system, with different levels of dexamethasone. According to FabRx, the devices were fabricated with good resolution and reproducibility, featuring a tapered shaft to allow for easy insertion and removal.
Taking just 20 minutes, more than 20 punctal plugs could be produced in a single print, apparently demonstrating the capability of 3D printing technologies for the fabrication of small-batch personalized drug delivery devices.
The researchers tested their 3D printed punctal plugs on an in vitro model created in-house that mimics the subconjunctival space of the eye. Complete prolonged drug release of the dexamethasone was achieved within four to 11 days, depending on the amount and formulation of the drug within the punctal plug.
For instance, the in vitro tests revealed that drug release from D10PEG and D20PEG punctal plugs could be sustained for a week, while D10 and D20 plugs could prolong the drug release with 50 percent of the dexamethasone released over five and 11 days respectively.
According to the partners, compared with traditional methods of preparing punctal plugs, such as molding, DLP 3D printing offered high flexibility in personalizing the size, shape, and material of the plug to suit different patients’ needs. The study also demonstrates the potential of drug-releasing punctal plugs to benefit a range of ocular diseases, including open-angle glaucoma, ocular hypertension, and bacterial conjunctivitis.
Further information on the study can be found in the paper titled: published in the MDPI Pharmaceutics journal. The study is co-authored by X. Xu, S. Awwad, L. Diaz-Gomez, C. Alvarez-Lorenzo, S. Brocchini, S. Gaisford, A. Goyanes, and A. Basit.

* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Hayley Everett

Leave A Comment