Chinese drug 3D printing firm Triastek has announced that its T22 3D printed gastric retention product has become the first drug of its kind to receive Investigational New Drug (IND) clearance from the US Food and Drug Administration (FDA).
T22 has been filed under section 505(B)(2) of the FDA’s Federal Food, Drug, and Cosmetic Act. The 3D printed drug is designed to treat pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH).
According to Triastek, this new product significantly reduces dosage frequency from three times a day to just once a day. This is said to simplify the dosing regimen and improve medication adherence.
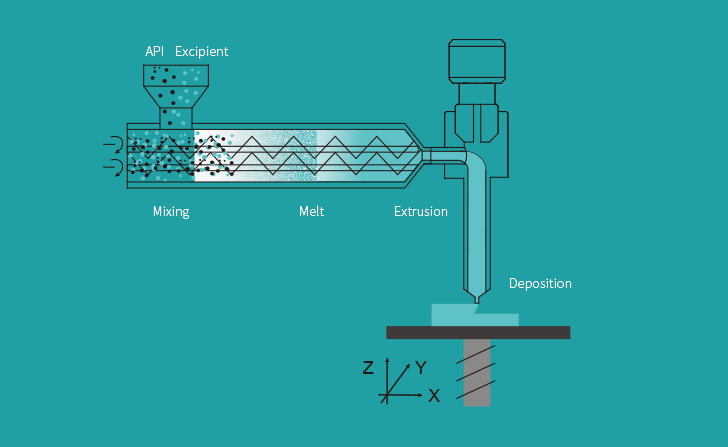
T22 is 3D printed using Triastek’s Melt extrusion Deposition plus Micro-Injection Molding (MED&MIM) process and incorporates its 3D Microstructure for Gastric Retention (3DµS-GR) for optimized drug delivery.
This is the fourth Triastek 3D printed drug to receive FDA clearance to the clinical development stage, adding to the company’s T-series portfolio of T19, T20 and T21 products. As such, the company claims to rank first in the global 3D printed drug field in terms of development product count.
Triastek is now preparing to initiate clinical trials with T22 to fast-track the product’s development.
“Based on our proprietary 3D Microstructure for Gastric Retention delivery technology platform, the two products we developed, T20G and T22, have received IND clearance to proceed from regulatory agencies in China and the United States this year, marking the successful first step for Triastek’s this innovative delivery technology platform proceeding through regulatory review process,” stated Triastek founder and CEO Dr. Senping Cheng.

MED 3D printed pharmaceuticals
Since launching in 2015, Triastek has built a business solely focused on the production of 3D printed solid dosage form drugs. Triastek boasts 213 patent applications related to its 3D printed pharmaceuticals across 10 countries, 68 of which have been granted.
The company’s proprietary MED extrusion-based 3D printing technology provides an end-to-end method for manufacturing a wide range of novel dosage form designs. The system continuously converts powder feedstocks into softened states. This molten material is then deposited layer-by-layer to produce physical drugs with optimized geometric structures.
Such structures would be otherwise impossible to produce. As such, the 3D printing process allows for drug release control at a level that can’t be replicated with conventional tablet production methods.
T22: Triastek’s newest 3D printed drug
Back in 2021, Triastek signed a co-development agreement with Sperogenix Therapeutics to develop and commercialize the T22 in East Asia. This partnership sought to demonstrate the clinical application value of 3DµS-GR.
Manufacturing on Demand
According to Dr. Cheng, the subsequent progress of the T22 has led to companies from several countries and regions to express interest in potential collaborations for product development utilizing the 3DµS-GR drug delivery platform.
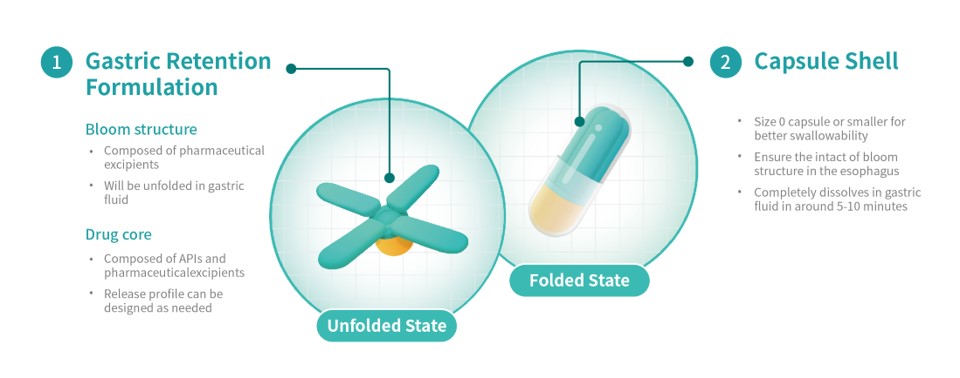
Triasktek has now submitted a Patent Cooperation Treaty (PCT) application for its 3DµS-GR delivery technology, and the unique Bloom Structure design of the T22.
Following oral administration, the T22 gastric retention prototype expands to a size larger than the pylorus, the opening from the stomach to the small intestine. This extends gastric retention time. During the gastric retention period, the T22 releases APIs according to predetermined programmed drug release behavior.
In addition to simplifying the dosage regimen, reducing medication burden, and improving long-term medication adherence, this process can also improve drug absorption and oral bioavailability.
Triastek has now completed development of the T22 gastric retention formulation. This formulation is said to have achieved positive results in terms of in vitro expansion time, mechanical strength and dissolution behavior.
The company has also completed Pharmacokinetic (PK) studies of the T22 prototype on beagle dogs. The PK study assessed how the body interacts with the drug for the duration of exposure. Here the company demonstrated that one daily dose of the T22 prototype gave comparable PK parameters as three times a day dosing of the original product.
Developments in pharmaceutical 3D printing
In recent surveys on the future of 3D printing and 3D printing trends, 3D printing experts highlighted advancements in medical applications as being a key area of current industry growth that will continue in the coming years.
Indeed, Triastek is not the only company to make notable advancements in the 3D printing pharmaceutical drug delivery space.
Last year, a research team from the Max Planck Institute for Informatics in Saarbrücken, Germany, and the University of California at Davis developed novel 3D printed pills that can release pharmaceutical drugs at predetermined speeds. In a research paper, the team demonstrated how the pills can be 3D printed with specific shapes which determine the speed at which they dissolve in the human body.
Given that geometric shapes are easier to control than alternative timed drug-delivery methods such as intravenous infusion, the researchers claim that this new method possesses significant potential beyond pharmaceutics. For instance, the team claims that the production of catalytic bodies and coarse granular fertilizers may be additional applications of their findings.
Similarly, in 2016 Aprecia Pharmaceuticals announced that their Spritam medication had become the first 3D printed pharmaceutical to receive FDA approval. Spritam is designed to treat a range of seizures and is 3D printed as an instantly dissoluble tablet. This makes the medication more accessible to patients who would otherwise struggle to swallow the pill.
You might also like:
Open Bionics’ new 3D printed fingers adopted for the first time, commercial launch coming soon: The new 3D printed prosthesis, called the Hero Gauntlet, was made for 50-year-old Londoner Suleman Chohan using Open Bionics’ 3D scanning and additive manufacturing technology.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Alex Tyrer-Jones

Leave A Comment