Bioprinting company T&R Biofab and B Braun, a major healthcare solutions provider, held a joint presentation to launch T&R’s new 3D printed PCL Craniofacial bone implants products. The event, hosted by B Braun Korea was held at the Dragon City Hotel in Seoul.
During the event, T&R Biofab outlined its future business vision through craniofacial bone-implant products and its strategies to enter the domestic and foreign markets. Led by President and CEO Wonsoo Yun, T&R has experienced rapid growth in recent years leveraging a unique 3D printing and bioprinting technology. “We will now strategically expand the domestic and overseas markets through collaboration with B Braun Korea,” said Wonsoo Yoon, adding that B Braun has an excellent sales network.
T&R Biofab researches, develops and manufactures printing cell therapeutics. The company has developed systems to produce biodegradable medical devices based on 3D bioprinting technology, as well as bioinks for tissue and organ bioprinting, organoids for in vitro testing.

Manufacturing on Demand
In 2020, T&R Biofab and B Brown Korea signed an MOU on joint development and sales of products for tissue regeneration and treatment in the neurosurgery sector, and have since been conducting extensive R&D. As per the agreement, T&R Biofab will produce and supply the Cranifacial bone-Implants while B Braun Korea will cooperate with local agencies to distribute and sell the products.
The T&R Craniofacial Implant is a product is made of special surgical material for reconstructing and repairing cranial trauma or defects that occurred during craniotomy in neurosurgery. In the past, a material called bone cement (also known as hydroxyapatite) was used to fill and join defects during surgery, in parts such as the skull, but it had limitations in terms of durability and was known for causing inflammation and infection. Therefore, in the field of neurosurgery, the demand for products that can replace existing surgical materials has continued.
More specifically, T&R focuses on two applications. One is gap filler for the bone flap in “large” craniotomy operations. As bone cement is very fragile, custom-made prostheses are known to have complications at rates which are above 40% of the cases, often due to fracture. The main advantage of using bone cement is not relative to its efficacy (since efficacy could be even lower, with higher prices), but rather to the cosmetic effect. The T&R Craniofacial implants bypass this situation.
The second application is as a cap for covering the burr hole of craniotomy, EVD (extra ventricular drain) and tumor biopsy operations. Patient satisfaction with the application of a cover for the burr hole dramatically increases (as shown by a study on titanium plugs). The ease of application and manipulation, if needed, during surgery and the possibility of the patient’s own bone regrowth give PCL plugs a significant advantage.
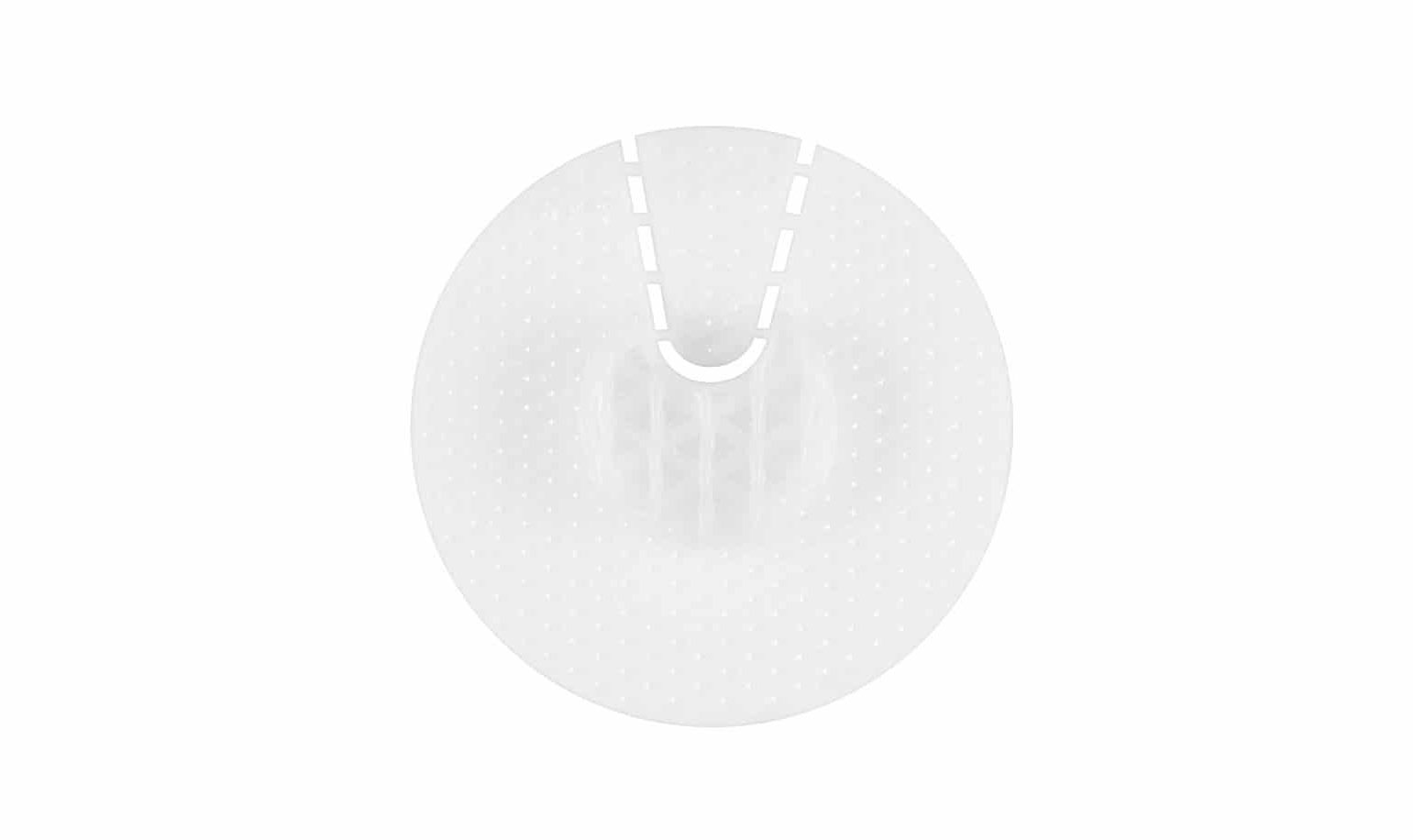
The new Craniofacial-bone Implant products are made of biodegradable PCL materials to induce reconstruction and restoration of damaged skull tissue and effective tissue regeneration after surgery. For these benefits, T&R Biofab received very positive feedback from the medical community in previous academic seminars. In total, the company is commercializing five types of implants: mesh (plate), three different size caps for burr hole (EVD cap) and one interconnectable (gap-filler) unit.
The company estimates that the Asian market for neurosurgical materials is very significant in China as well as Japan, and other Asia-Pacific regions. The two companies are now planning to expand their partnership with various other 3D bioprinting products, starting with neurosurgery materials.
* This article is reprinted from 3D Printing Media Network. If you are involved in infringement, please contact us to delete it.
Author: Davide Sher



Leave A Comment