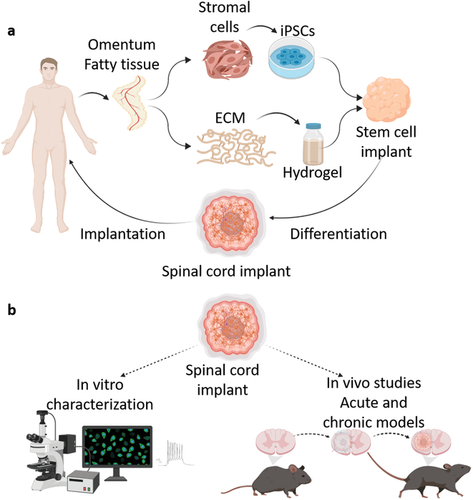
3DBio Therapeutics reconstructs human ear using bioprinted living tissue Bioprinting
3DBio Therapeutics, a clinical-stage regenerative medicine company, and the Microtia-Congenital Ear Deformity Institute have conducted a human ear reconstruction using the AuriNovo implant – an investigational, patient-matched, 3D bioprinted living tissue ear implant.