Regenerative medicine specialist CollPlant (NASDAQ: CLGN) has revealed that the 3D bioprinted breast tissues it’s developing are set to enter animal trials in the very near future.
Alongside the release of its FY 2021 results, which show that it generated $15.6 million in revenue, 155.7% more than the $6.1 million it reported in FY 2020, CollPlant announced that its bioprinted implants will enter in-vivo testing from Q2 2022.
Currently being developed with BICO firm CELLINK, the grafts are designed to gradually degrade and be replaced by native tissues, in a safer alternative to breast augmentation procedures. With further R&D, the companies believe their approach could be deployed in up to 2.2 million surgeries, enabling them to address a market worth an estimated $2.8 billion.
“In the year ahead, we anticipate reaching development milestones for some of our leading programs, including the start of a large animal study for our 3D bioprinted regenerative breast implant program,” said Yehiel Tal, CEO of CollPlant. “We are also making continued progress with the development of bio-inks for 3D bioprinting applications, and with development of a photocurable dermal filler.”

CollPlant’s FY 2021 financials
Although CollPlant hasn’t provided a division-by-division breakdown of its annual revenue, it’s clear from what has been revealed, that its rate of growth is beginning to accelerate. In large part, the firm’s rapid rise in revenue last year was down to a $103 million bioprinting contract it signed with AbbVie company Allergan Aesthetics during March 2021.
As part of this agreement, the latter has begun to use CollPlant’s artificial collagen to create dermal and soft tissue fillers, and it could go on to develop two more products in future. The deal has also seen CollPlant receive an initial $14 million, and while this represented 90% of its FY 2021 revenue, it could gain another $89 million in milestone payments should any of Allergan Aesthetics’ products make it to market.
In addition to generating more revenue in FY 2021 than FY 2020, the company improved its profitability as well, raising its gross profit from $3.1 million to $13.6 million. This was primarily due to the fact that over this period, its cost of revenue fell from $3 million to $2 million, owing to expenses related to royalties, bio-ink and rhCollagen sales, and the ending of its deal with United Therapeutics in FY 2020.
Thanks largely to a registered direct offering in February 2021, which saw it raise $31.8 million in net proceeds and $6 million from the exercise of warrants, CollPlant was able to attract $38.8 million in financing across last year. As it happens, some $31.6 million of this funding was deployed immediately in short-term cash deposits, the likes of which generated up to $172,000 for the firm in FY 2020.
| CollPlant Financials ($) | FY 2020 | FY 2021 | Difference (%) | FY 2019 | FY 2021 | Difference (%) |
| Revenue | 6.1m | 15.6m | +155.7 | 2.3m | 15.6m | +578.3 |
| Cost of Revenue | 3m | 2m | -33 | 1.9m | 2m | +5.3 |
| Gross Profit | 3.1m | 13.6m | +338.7 | 0.4m | 13.6m | +3300 |
| Net Cash From Financing Activities | 4.5m | 38.8m | +762.2 | 5.4m | 38.8m | +618.5 |
| E.O.Y Cash Balance | 3.5m | 13.4m | +282.3 | 4m | 13.4m | +235 |
CollPlant’s breast implant trials
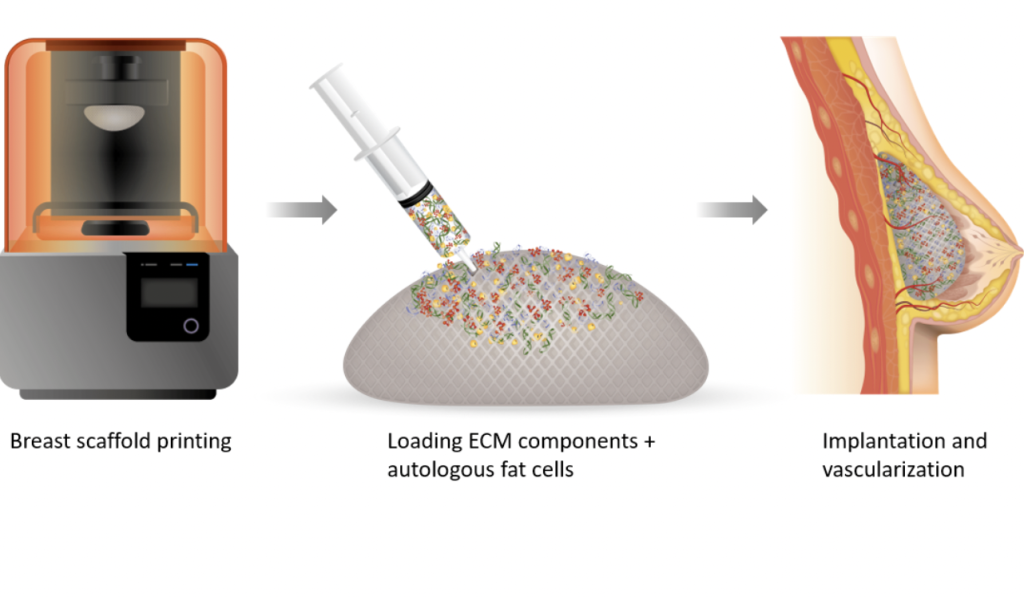
Since announcing the creation of its first regenerative breast implantsin 2019, the firm has steadily sought to improve their clinical and commercial viability. The tissues themselves are made from patient fat cells and ECM components, as well as ‘rhCollagen,’ CollPlant’s tobacco plant-grown alternative to animal or cadaver-sourced collagen.
Manufacturing on Demand
In theory, once these grafts are injected into patients’ tissues, they foster the regeneration of native cells before slowly degrading, leaving behind no foreign contaminants. CollPlant says this procedure could offer a “revolutionary alternative” to silicone implants or fat transfer operations which come with a ‘risk of adverse events,’ but they aren’t yet ready for market, and continue to undergo clinical trials.
One way the company has sought to accelerate the development of its bioprinted breast implants is via its recently-established partnership with CELLINK. When the collaboration was announced last month, CollPlant said that CELLINK’s “high-throughput bioprinters” and expertise could enable it to overcome the hurdles facing its implant’s scalability.
That being said, the firm has also worked with 3D Systems before to develop breast reconstruction treatments for cancer survivors, and it’s possible that its current work will allow it to build on some of the findings it made as part of this project too.
In the case of its newly-announced trials, CollPlant has let little slip about their exact set up, but they’re understood to offer an opportunity to put its learnings from preclinical studies into practice, while taking a significant step forward in its breast implants’ R&D, in that their viability will now be tested in-vivo at scale.
Advances in breast implant bioprinting
Even though 3D bioprinting itself remains an emerging technology, a significant amount of progress has already been made in using it to produce viable breast implants. Earlier this year, Healshape raised $6.8 million towards the R&D of its patient-specific breast tissuesdesigned to treat those who have undergone a mastectomy.
In the past, Plcoskin has also announced plans to work with Yonsei University and LipoCoat with the aim of coming up with a novel 3D printed breast implant. In essence, the project was set up to combine LipoCoat’s lipid film coating technology and Plcoskin’s PCL-collagen coating approach, as a means of developing a uniquely-glazed graft that offers a reduced risk of infection or rejection.
Elsewhere, similar technologies are being developed to create all sorts of other tissues as well, ranging from the 3D bioprinted human tescticle cells produced at the University of British Columbia, to the bioprinted liver tissues of T&R Biofab.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment