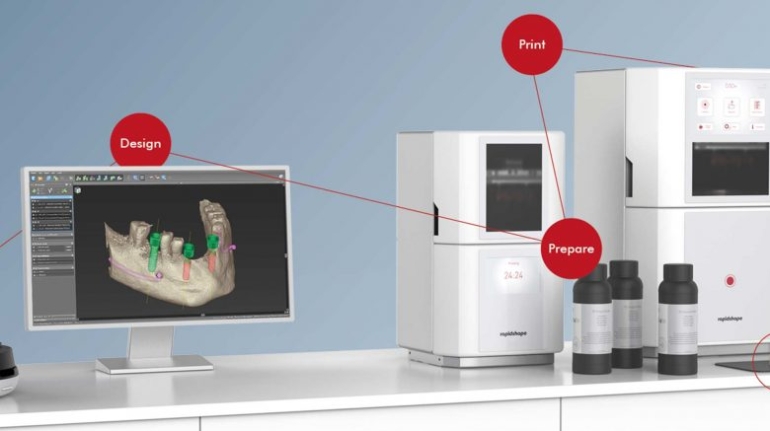
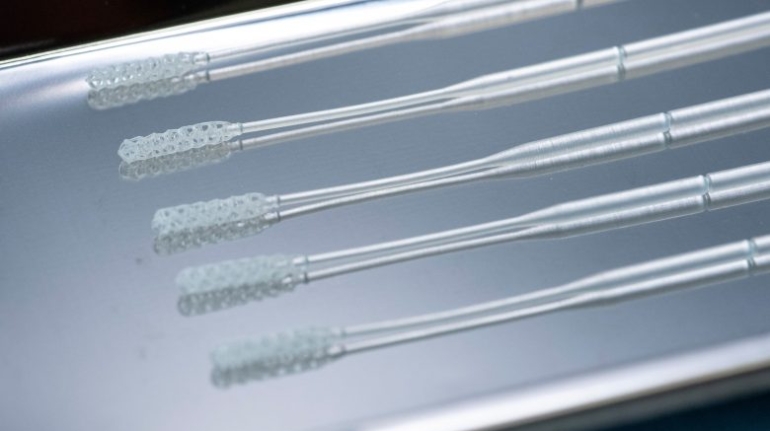
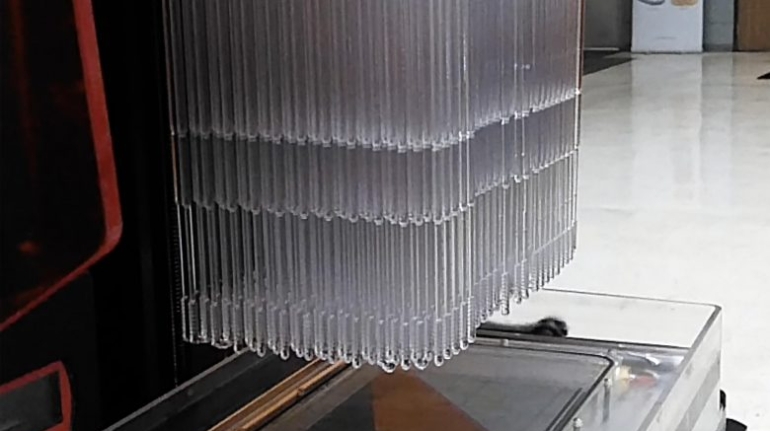
Rapid Shape and VOCO qualify dental materials for 3D printing Medical
German additive manufacturing company Rapid Shape has announced a partnership with dental manufacturer VOCO, also based in Germany. The companies are combining their capabilities—including 3D printing systems and dental-grade materials—to offer their customers more freedom and flexibility when choosing what materials to work with. In short, the companies will work to qualify more dental materials for additive manufacturing.