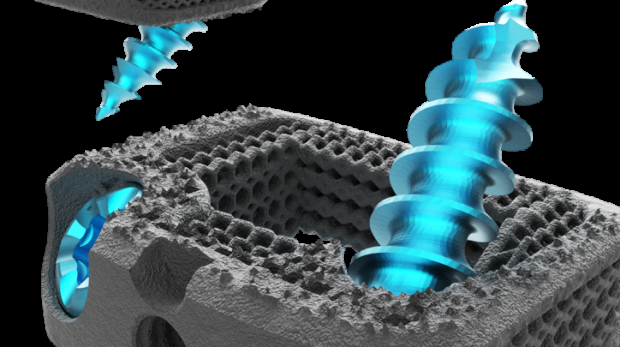
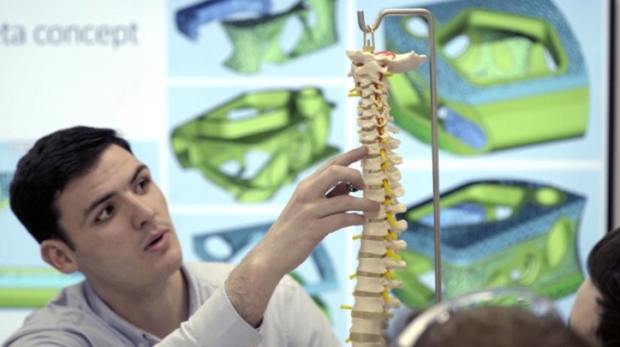
How medical device companies use AM for production today part 1: Stryker additive manufacturing Medical
Welcome to this month’s AM Focus Medical. For the entire month of February, we are going to zoom in on the many possibilities that additive manufacturing is offering today to medical companies. This segment of AM is literally booming and incredibly exciting. In this first episode, we are taking a closer look at Stryker additive manufacturing activities. Upcoming episodes will include many different types of players in this segment, ranging from highly innovative startups to giant multinational corporations. Stay tuned because it’s going to be a lot to take in. But don’t worry, at the end of the month all the best content will be featured in 3dpbm’s Medical AM Focus 2020 eBook.