Using 3D printers, researchers have collaborated from around the globe to develop nanoclay-based 3D bioprinted scaffolds which could be used to aid skeletal regeneration.
Hailing from the University of Southampton, the Istituto Italiano di Tecnologia in Rome, University Hospital Carl Gustav Carus and Technische Universität in Dresden, and China Medical University in Taiwan, the researchers 3D bioprinted implantable nanocomposite scaffolds, laden with human bone marrow stromal cells (HBMSCs) and human umbilical vein endothelial cells (HUVECs), which have the potential to facilitate bone formation.
3D bioprinting for orthopedics
Ideally, 3D printed implants of this nature should sustain cell viability and promote cells to multiply, in addition to generating functional constructs shortly after printing and stimulating the host microenvironment to aid tissue growth.
Realistically, bioprinting is in its infancy and the prospect of whole 3D printed transplant organs is still probably decades away. However, there have been a number of innovative developments in the field thus far, such as a novel bio-ink enabling scientists at the University of Minnesota to create a functional 3D printed beating human heart.
Similarly, researchers from Tsinghua University have 3D bioprinted brain-like tissue structures capable of nurturing neural cells, while in May microdispensing specialist nScrypt and aerospace company TechShot successfully completed the first functional 3D bioprinting experiment in space – a human knee meniscus.
Most recently, researchers from the University of Montreal have developed a new method of cell bioprinting based on a drop-on-demand technique, called Laser Induced Side Transfer, which utilizes a low energy nanosecond laser and the laws of microfluidic dynamics to jet living cells onto each other. The team believes their work could be adapted for applications such as 3D drug screening models and artificial tissues.

The nanoclay-based method
According to the report, nanoclay-based bioink formulations are particularly attractive for implant applications given their ability, even at low concentrations, to shear while being extruded and regain their shape upon deposition, while shielding cells from potential damage from the printing process.
During the study, scientists harnessed the physiochemical properties of Laponite (LAP), a smectite nanoclay suspension, and combined it with HBMSCs, bone morphogenic protein-2 (BMP-2), and vascular endothelial growth factor to produce LAP-alginate-methylcellulose bioink. HBMSCs, collected from patients undergoing routine hip surgery, and HUVECs, obtained from the umbilical cords of healthy mothers after normal, full-term deliveries, were encapsulated in the bioink and printed using an in-house built bioprinter.
Manufacturing on Demand
After printing, the scaffolds were incubated for 10 minutes in a sterile calcium chloride solution to enable crosslinking.
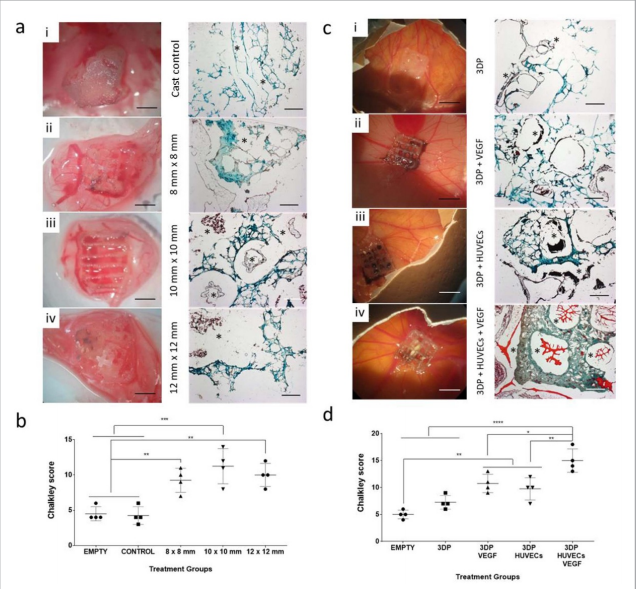
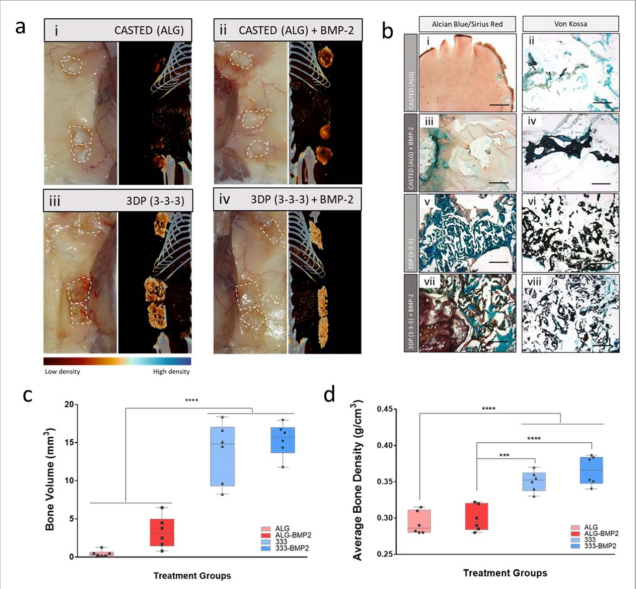
The skeletal functionality of the HBMSCs-laden 3D bioprinted scaffolds was investigated in vitro, ex vivo, and in vivo. The results demonstrated significant improvements in mineralized tissue formation with the addition of HBMSCs in 3DP, but not in mold-cast bulk scaffolds.
Significance of the findings
According to the researchers, the printing of bioinks laden with cells that can act as building blocks for the generation of tissue-like structures represents a simple and effective approach to produce readily implantable constructs.
The potential to print stem cells, preserving cell viability, proliferation, and functionality, is currently a key unmet challenge for the biofabrication approach to regenerative medicine. Clay-based bioinks, such as the one looked at in this study, are now proven to offer an attractive vehicle for printing HBMSCs in three-dimensional constructs due to their shear-thinning and inherent functional properties.
Further details of the study can be found in the article titled , published in the Biofabrication journal. The article is co-authored by Gianluca Cidonio, Michael Glinka, Yang-Hee Kim, Janos Kanczler, Stuart Lanham, Tilman Ahlfeld, Anja Lode, Jonathan Dawson, Michael Gelinsky, and Richard Oreffo.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Hayley Everett

Leave A Comment