Researchers from the Technical University of Munich (TUM) and the University of Western Australia are developing 3D printed artificial heart valves made from a patient’s own cells that grow as the individual ages.
The approach hopes to overcome the drawbacks of conventional prosthetic heart valves, which only last a limited number of years and therefore require multiple replacement surgeries.
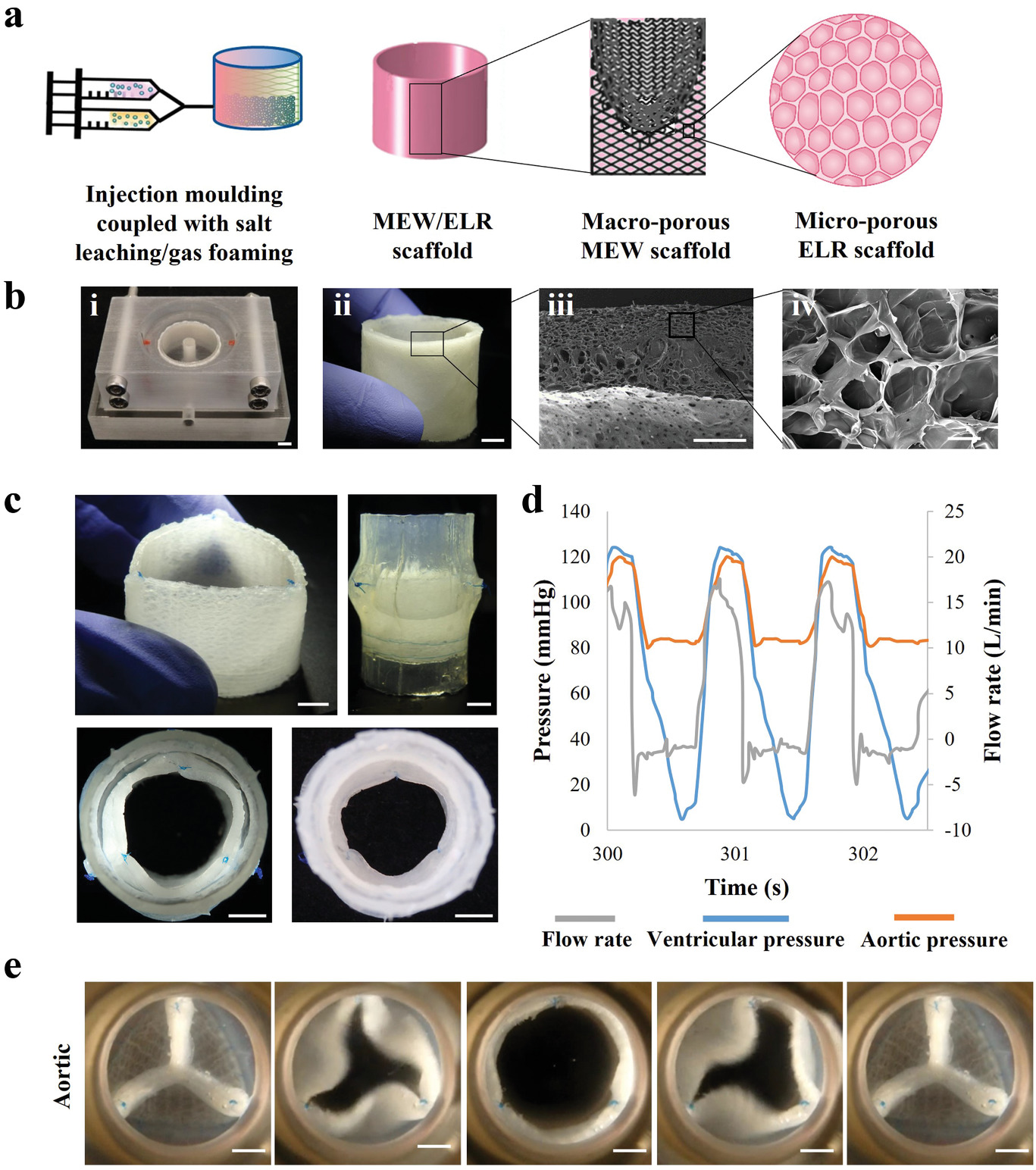
Lead by TUM professor Petra Mela and Elena De-Juan-Pardo of the University of Western Australia, the team leveraged a melt electrowriting 3D printing process to create porous scaffolds made up of a patient’s own cells which can grow as the patient does.
Melt electrowriting 3D printing
Melt electrowriting is an advanced additive manufacturing technology capable of depositing predefined micrometric fibers, and works by combining an applied electric field, temperature, and pressure to create a charged jet of molten polymer.
The researchers used the technique to deposit microfibers less than one-tenth the thickness of a human hair with extreme precision in a predefined pattern, giving the resulting fibrous scaffolds “excellent” features.
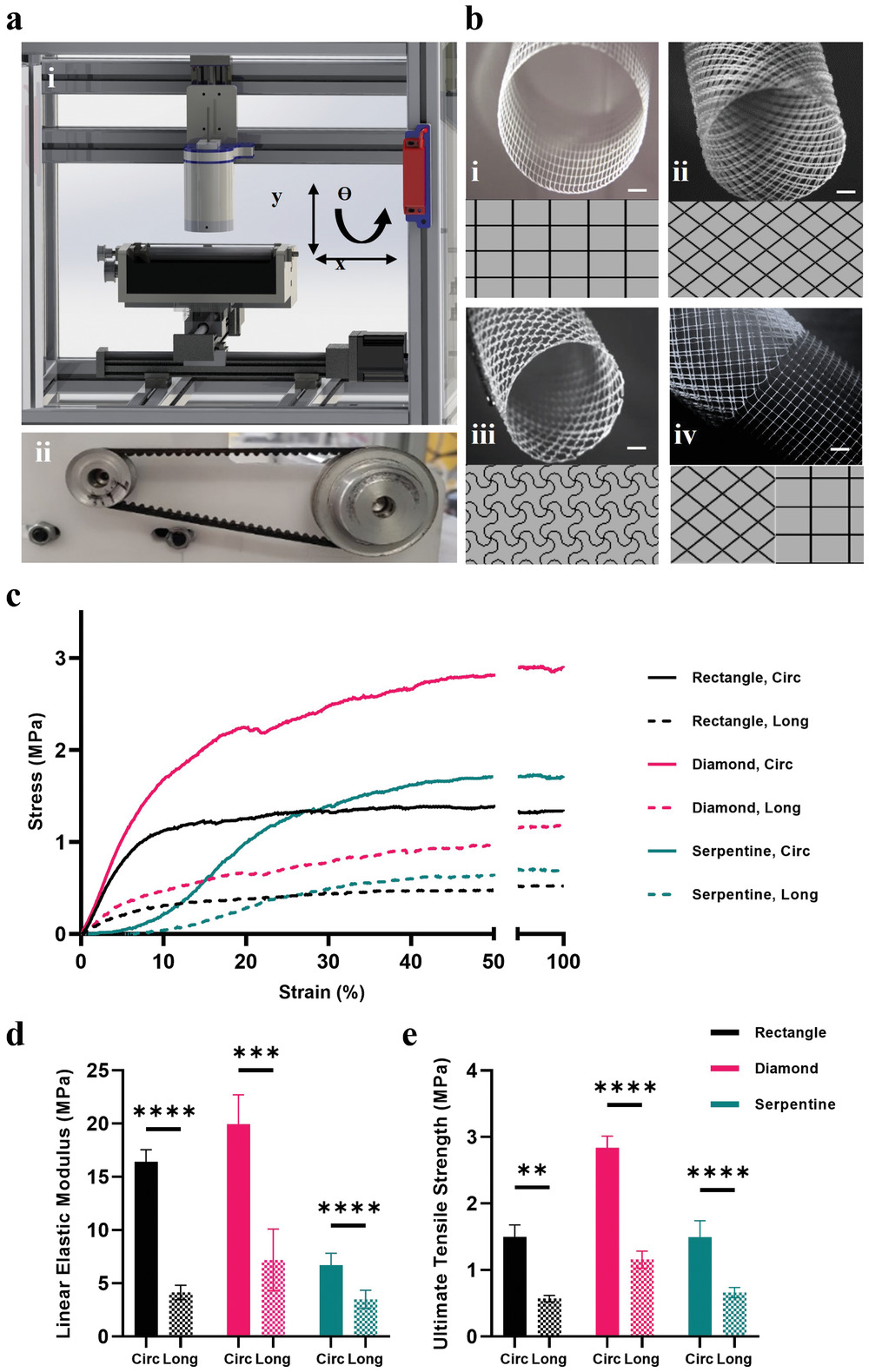
Melt electrospinning provides significant advantages over other fiber-forming techniques such as conventional electrospinning, as it enables the fabrication of scaffold with tunable mechanical properties, macro-porosities, and patterns for a wide range of applications, including implant tissue engineering and disease modeling.
The technique has been deployed within a biomedical context before, having been used by MIT to grow highly uniform cell cultures with particular characteristics and by the University Medical Centre (UMC) Utrecht to produce 3D bioprinted tissues that can be implanted into a living joint affected by arthritis.
Melt electrowriting also powers a 3D printer with “eyes and brains” developed by the Queensland University of Technology, which integrates artificial intelligence (AI) and machine learning (ML) to manufacture customized medical implants.
3D printing artificial heart valves
According to the World Health Organization (WHO), cardiovascular diseases are the leading cause of death globally, with valvular heart disease being the third leading contributor to cardiovascular disorders worldwide.
Currently, if damaged heart valves cannot be repaired, current treatments involve implanting a prosthetic valve which should ideally remain in the patient for the whole of their lifetime. However, such valves have a limited lifespan and so patients must undergo multiple surgical interventions to replace them. This problem is particularly prevalent in young pediatric patients, as they require new valves as their bodies grow.
The research team’s regenerative approach to heart valve tissue engineering sought to overcome the limitations of current mechanical and biological valve prostheses by fabricating a valve with the ability to grow and remodel with the patient. To achieve this, they needed to print the scaffolds with adequate porosity to enable the cells to infiltrate the structure and thrive.
Manufacturing on Demand
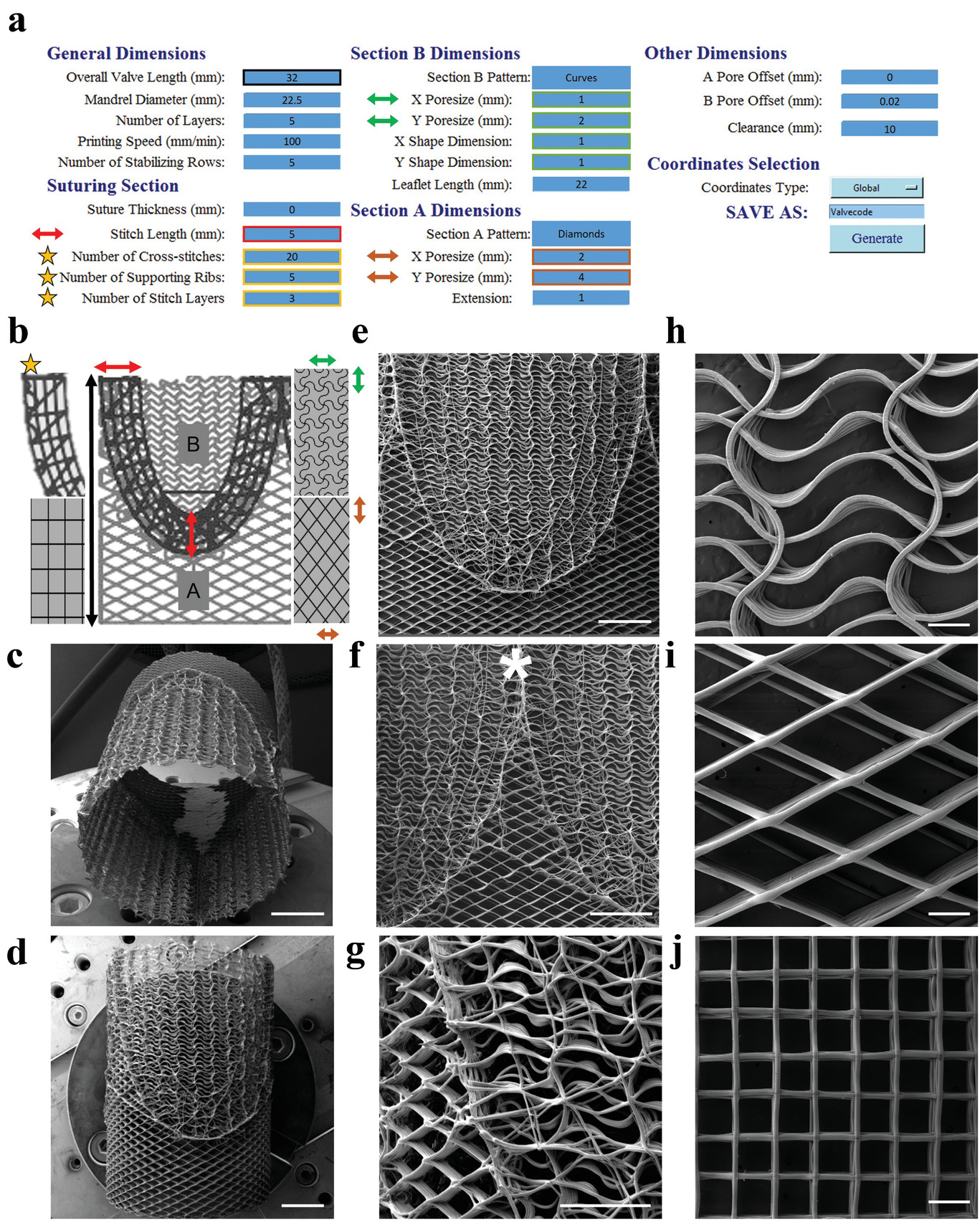
The team used an in-house melt electrowriting 3D printer to create heart valve implants that mimicked the various tissue structures of an individual patient’s own aortic heart valve. The team’s digital platform 3D printed complex patterns which were then composited with tubular microporous hydrogel scaffolds.
The resulting printed structure was capable of withstanding the demanding functions of a heart valve while remaining porous enough to allow the patient’s own cells to colonize the scaffold and proliferate. The team tested the capabilities of their artificial heart valve by creating a mock circulatory system and subjecting it to the same pressure and flow rates that a natural heart valve would endure.
According to the team, the results of the testing phase were promising, with the valve satisfying ISO standards. While the results were encouraging, the team acknowledges the tests are not predictive of the valve’s long-term functionality, for which in vivo studies will be conducted to evaluate the scaffold’s remodeling process and degradation rate, among other factors.
For now, the researchers see their 3D printed heart valves as demonstrating a “pioneering proof-of-concept” for an off-the-shelf complete heart valve construct that could pave the way for other soft tissue engineering applications.
Further information on the study can be found in the paper titled: published in the Advanced Functional Materials journal. The study is co-authored by N. Saidy, A. Fernandez-Colino, B. Heidari, R. Kent, M. Vernon, O. Bas, S. Mulderrig, A. Lubig, J. Rodriguez-Cabello, B. Doyle, D. Hutmacher, E. De-Juan-Pardo, and P. Mela.
As the capabilities of bioprinting have improved, additive manufacturing has been leveraged for similar heart-related regenerative medicine applications in the past.
Back in 2020, researchers from the University of Minnesota worked with medical technology firm Medtronic to develop customizable 3D printed patient-specific heart valve models. The models were designed to help surgeons better prepare for minimally-invasive surgical procedures in order to improve the outcomes for cardiovascular patients.
Around the same time, researchers from Carnegie Mellon University developed their own 3D bioprinting method to produce full-size human heart models for surgical training and planning applications.
More recently, researchers from the Chinese Academy of Sciences 3D printed a beating heart that remained alive for six months using a six-axis robotic arm converted into a 3D bioprinter. According to the team, the 3D printed cardiac tissue could demonstrate a feasible method of bioprinting functional tissues and organs in the future.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Hayley Everett

Leave A Comment