Nexxt Spine, an Indiana-based spinal implant manufacturer, has announced that it will develop 3D printed bone healing spinal implants with the help of MTS Systems Corporation, a global manufacturer and supplier of simulations and testing systems.
Alaedeen Abu-Mulaweh, Nexxt Spine Director of Engineering, said, “Given the highly nuanced nature of these intricate micro geometries, the MTS testing platform has been our go-to solution for quantifying and tailoring the associated biomechanical performance attributes from day one.”

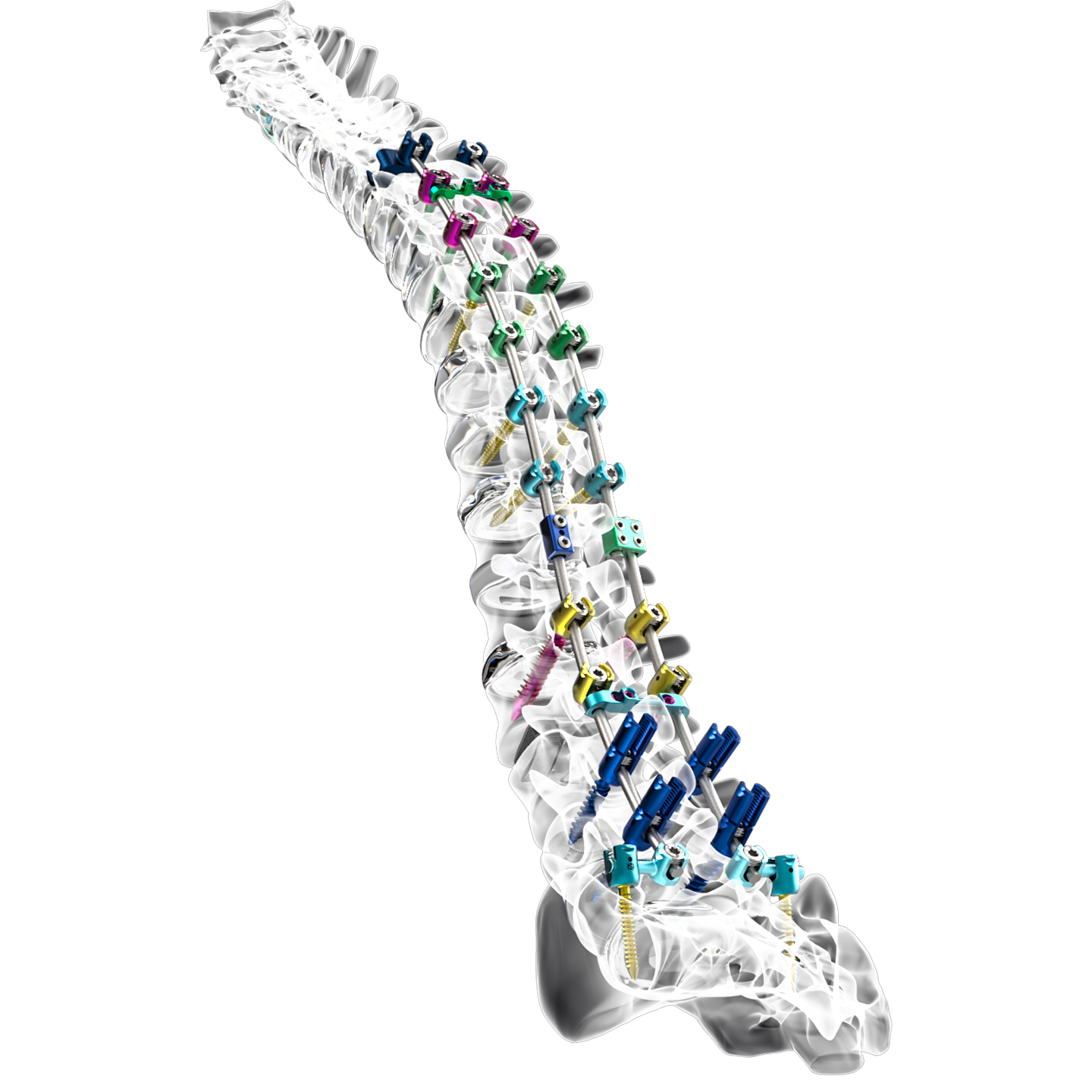
Nexxt Matrixx
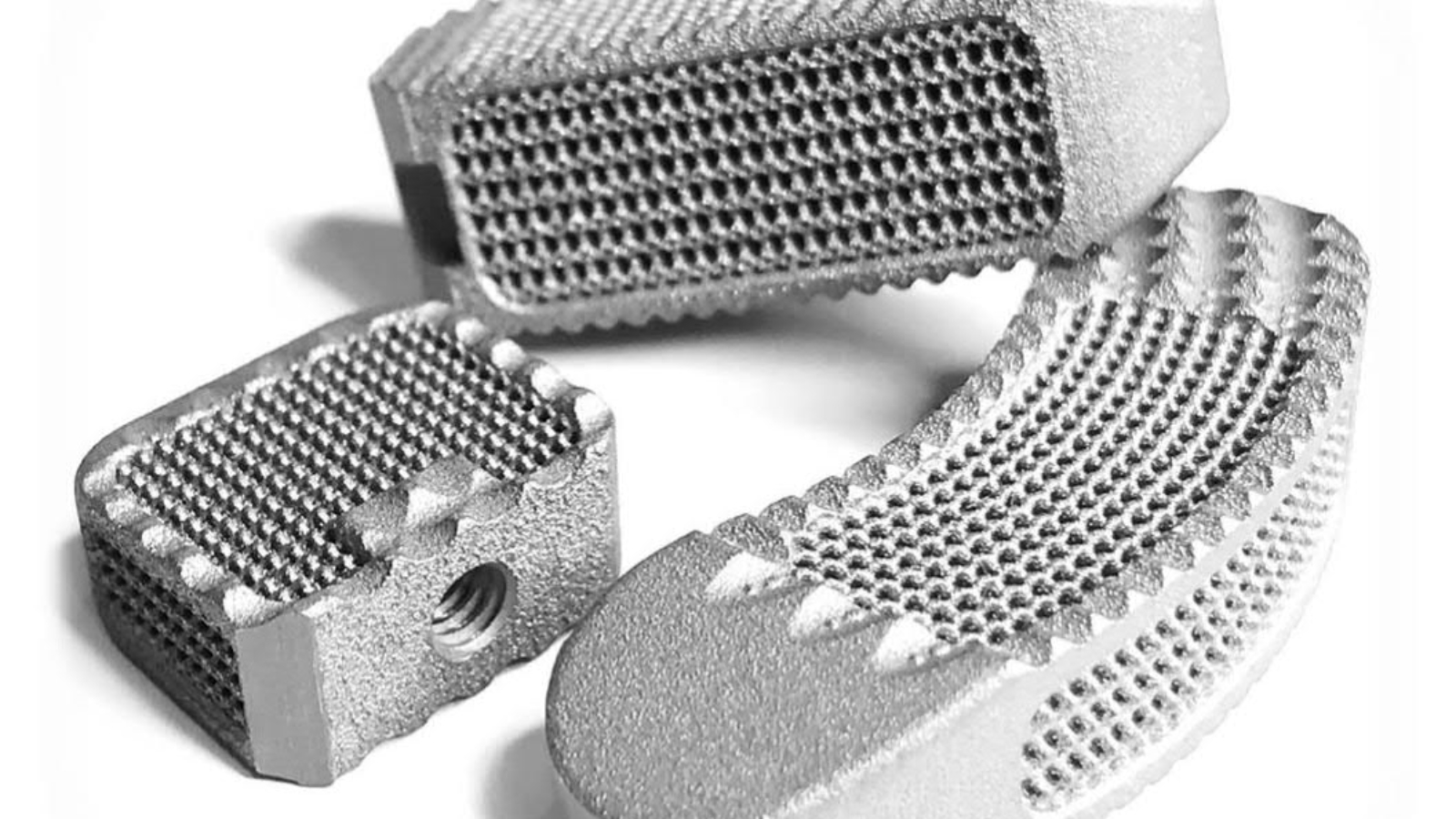
Nexxt Spine is known for developing innovative spinal implants and surgical guides. Among the company’s most popular products its proprietary FDA approved Nexxt Matrixx line of implants. These are porous structures 3D printed in titanium.
The Matrixx line of spinal implants includes Vertebral Body Replacement, TLIF/TLIF Oblique Interbody, and Cervical Interbody. According to the company over a thousand of Next Matrixx implants have been used by surgeons all over the United States.

Biomedical testing equipment
MTS is active in various sectors such as the aerospace, civil engineering, and biomedical industry. In the biomedical sector, the company is known for its Bionix Spine Kinematics System, a modular mechanical testing platform for spine kinematics research.
For the development of Nexxt Spines’s bone healing implants, MTS will provide its test systems for the purpose of quality assurance and part validation.
On the collaboration with Nexxt Spine, MTS President and CEO, Dr. Jeffrey Graves, commented, “MTS is pleased to support medical device manufacturers in their quest to design innovative products that improve health and well-being.”
Manufacturing on Demand
“In this case, MTS material test systems are helping Nexxt Spine find better ways to repair spines and improve quality of life for spine surgery patients.”
3D printed spinal implants
In recent year, the use of 3D printing in the biomedical industry has been very prolific. One of the reasons behind this is the publication of additive manufacturing guidelines by the FDA. In this context, the use of additive manufacturing in spinal surgery is particularly noticeable. This is in part because 3D printers can manufacture complex details at the micro level.
On the use of 3D printing by Nexxt Spine, Abu-Mulaweh, said, “We’ve immersed ourselves in the additive manufacturing space completely and positioned our future business focus as pioneers in the design and development of spinal fusion implants that incorporate interlaced micro-lattice architectures with the goal of promoting osteoconduction, osteointegration and bony fusion.”
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Umair Iftikhar

Leave A Comment