Over the past few decades, there has been a growing focus in the healthcare industry on achieving better patient outcomes. This emphasis is increasing the popularity of personalized medical devices, which are designed to match the anatomy or physiology of each patient.
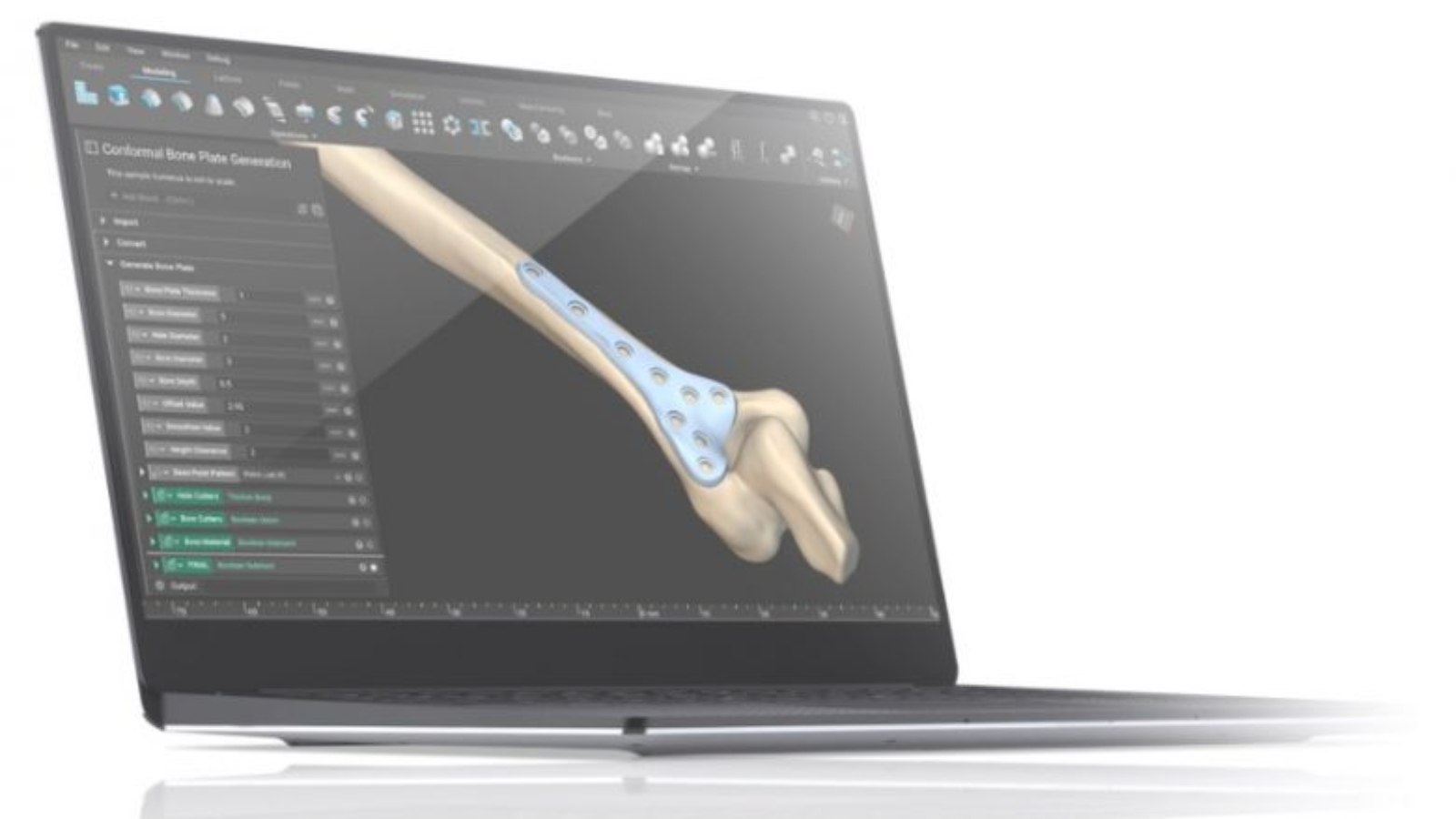
Examples of personalized medical devices are orthopedic implants that match the contour of the patient’s bone or prosthetic leg sockets featuring dynamic stiffness optimized to the patient’s lifestyle and body mass index.
Personalized medical devices provide an alternative to devices in standard sizing and have several advantages. They can shorten procedures and reduce the cost of care, improve operational precision to minimize the need for alterations, improve functionality and aesthetics, and increase the contact area to improve load distribution and reduce discomfort.
As companies turn to personalized medical devices to improve patient outcomes, advanced technologies like additive manufacturing (AM) and medical imaging are becoming more necessary. This article explores how to design personalized medical devices, covering the challenges, the benefits of additive manufacturing, and other advanced technologies that can support the design and manufacturing process.

Additive manufacturing for personalized medical devices
Traditional manufacturing technologies have a variety of limitations that can make the production of personalized medical devices difficult or even impossible. On the other hand, additive manufacturing offers a range of benefits that make it ideal for creating custom medical devices.
AM technologies enable manufacturers to create automated, scalable, end-to-end workflows that allow them to develop customized products with high accuracy and precision quickly. Not only does this allow companies to provide effective treatments tailored to individual patients, but it also shortens their product development cycle.
Furthermore, AM allows you to create biologically-relevant structures that mimic actual tissue more accurately than competing manufacturing methods. Thanks to its ability to accelerate product development, AM enables manufacturers to keep pace with evolving market trends while maintaining high-quality standards. Accelerated product development also means that once you overcome the initial challenge of establishing a process, you can simply replicate it across different devices.
Synergistic technologies
When paired with other emerging technologies, additive manufacturing can unlock additional value for personalized medical devices.
- Diagnostics & Planning Software
When creating a personalization workflow, you must start with an accurate representation of the patient’s unique physiology and anatomy. Imaging software that can post-process CT scans and MRI data with machine learning can speed up surgical planning and improve segmentation accuracy. - Design Automation
Design can pose a financial challenge when scaling the production of patient-specific devices. With advanced design software, you can standardize and even automate repetitive tasks, which cuts costs, reduces errors, and minimizes time to delivery. - Robotic Surgery
Precise placement is critical when implanting personalized medical devices, particularly when conducting soft tissue surgery. According to many clinical studies, robotic-assisted surgery can improve accuracy.

Personalized medical device challenges
Patient-specific cranial bone plate with a shape that mirrors the undamaged part of the skull.
Medical device companies face a few challenges when developing personalized medical devices.
Materials
A key challenge faced by medical device companies creating personalized devices is the availability of suitable materials for AM. Some of the biocompatible materials available for AM include Titanium, Nylon, and PEEK. However, the materials available that are both sterilizable and biocompatible are still very limited.
Fortunately, you can overcome this limitation by taking advantage of the design freedom associated with AM and its ability to manufacture complex structures. In the case of medical applications, the most common AM processes are metal and polymer powder bed fusion, material extrusion, and vat photopolymerization. Deciding which process is most suitable will depend on your use case, the cost, accuracy, and desired material properties.
A great benefit of additive manufacturing is that it enables the creation of architected materials, which are highly-engineered structures with targeted performance characteristics. These materials allow you to treat various physical properties, such as impact absorption or stiffness, as design variables.
Manufacturing on Demand
By applying architected materials in the design and manufacturing of medical devices, you can develop structures that imitate biological organisms, resulting in more effective treatments.

Design Automation
The cost of customization is often a major consideration when it comes to additive manufacturing for personal medical devices. While the cost associated with customization for AM production is marginal, the cost of customization during the design phase can be substantial. This cost is due to the time and expertise required for skilled engineers to manually create personalized designs for each patient.
For a personalized medical device product to be financially viable, a high degree of design automation is required and can shorten development cycles and reduce the cost of personalization.
To maximize the benefits, design automation should be embraced at every stage of the process. During the initial R&D phase, design automation allows you to quickly explore the available design space, identify critical design variables, and lock in essential parameters. At the end of this phase, you should have an automated design workflow that can be utilized across various other designs, saving time and money.
In the development phase, your workflow should be ready to deploy in a production environment. With a Graphic User Interface (GUI), less experienced users — such as lab technicians — can run your automated design process using patient-specific data as inputs. They can then inspect the results and troubleshoot with relative ease.
Design automation via a programmatic environment facilitates economic feasibility when it is time to scale production. For instance, you can minimize manual design time by using scripts that run on a server or cloud and call upon design generation workflows.

Regulatory Compliance
Apart from the typical hurdles associated with validating these innovative technologies, personalized medical devices must also adhere to stringent quality control standards. These standards are often based on standardized device geometries and assumptions about typical clinical conditions. However, because the geometry of personalized devices can vary greatly from patient to patient, adhering to these standardized methods can be difficult.
Nonetheless, these challenges need not present an insurmountable obstacle for innovative companies, as regulatory bodies are becoming increasingly receptive to technological advances and are more willing to approve personalized devices.
Having a traceable design process is essential for developing safe and reliable products. A traceable design process provides a digital trail that captures all the decisions and input parameters that define the nature of the product. This process enables regulatory review of design submissions. A traceable design also improves confidence in the product by documenting development risks, ensuring corrective actions can be executed if needed, and lending credibility to a personalized medical device.
Best practices for designing for traceability include:
-
- Unique file naming to keep track of design variations
- Exporting key metrics and design metadata of design outputs and inputs as a text file
- Adding serial numbers or marks to help you identify the physical device during production
- Automatically generating test coupons for quality control and validation for each design to eliminate a manual design step
- Design Software for Personalized Medical Device Design
Design software for personalized medical devices design
Topology is next-generation advanced engineering design software built from the ground up to enable you to maximize the benefits of additive manufacturing. This software gives you design features to develop innovative personalized medical devices that improve patient outcomes, reduce costs, and facilitate personalization at scale.

Download the Personalized Medical Devices Guide
Are you interested in learning more about personalized medical devices? Download nTopology’s comprehensive Personalized Medical Devices Guide to read case studies and learn about the digital workflows and applications for personalized medical devices.
This article was kindly brought to you by:

You might also like:
Desktop Health receives FDA clearance for SmileGuard resin: Among the previously-available 3D printed bite sprint solutions in the marketplace, the strongest materials are often uncomfortable to patients, while softer materials are often not as durable. SmileGuard resin solves this challenge.
* This article is reprinted from 3D Printing Media Network. If you are involved in infringement, please contact us to delete it.
Author: 3D Printing Media Network

Leave A Comment