Medical device manufacturer 3D LifePrints has gained ISO certification for the quality assurance procedures behind its 3D printed surgical guides and anatomical models.
Specifically, the ‘Embedmed’ Quality Management System (QMS) used by the firm to ensure the high standard of its medical products, has been recognized as ISO 13485:2016-compliant. Having met the accreditation’s stringent quality guidelines, 3D LifePrints can now continue offering its patient-specific device range, while weighing-up a potential expansion to its clinical portfolio.
“It has long been our goal to standardize our processes by adherence to this internationally-recognized standard,” said Henry Pinchbeck, CEO of 3D LifePrints. “The formal certification of our QMS opens up considerable market opportunities for the business, and is a clear signal to our customers of the importance we attach to quality assurance and patient care.”

3D LifePrints’ clinical approach
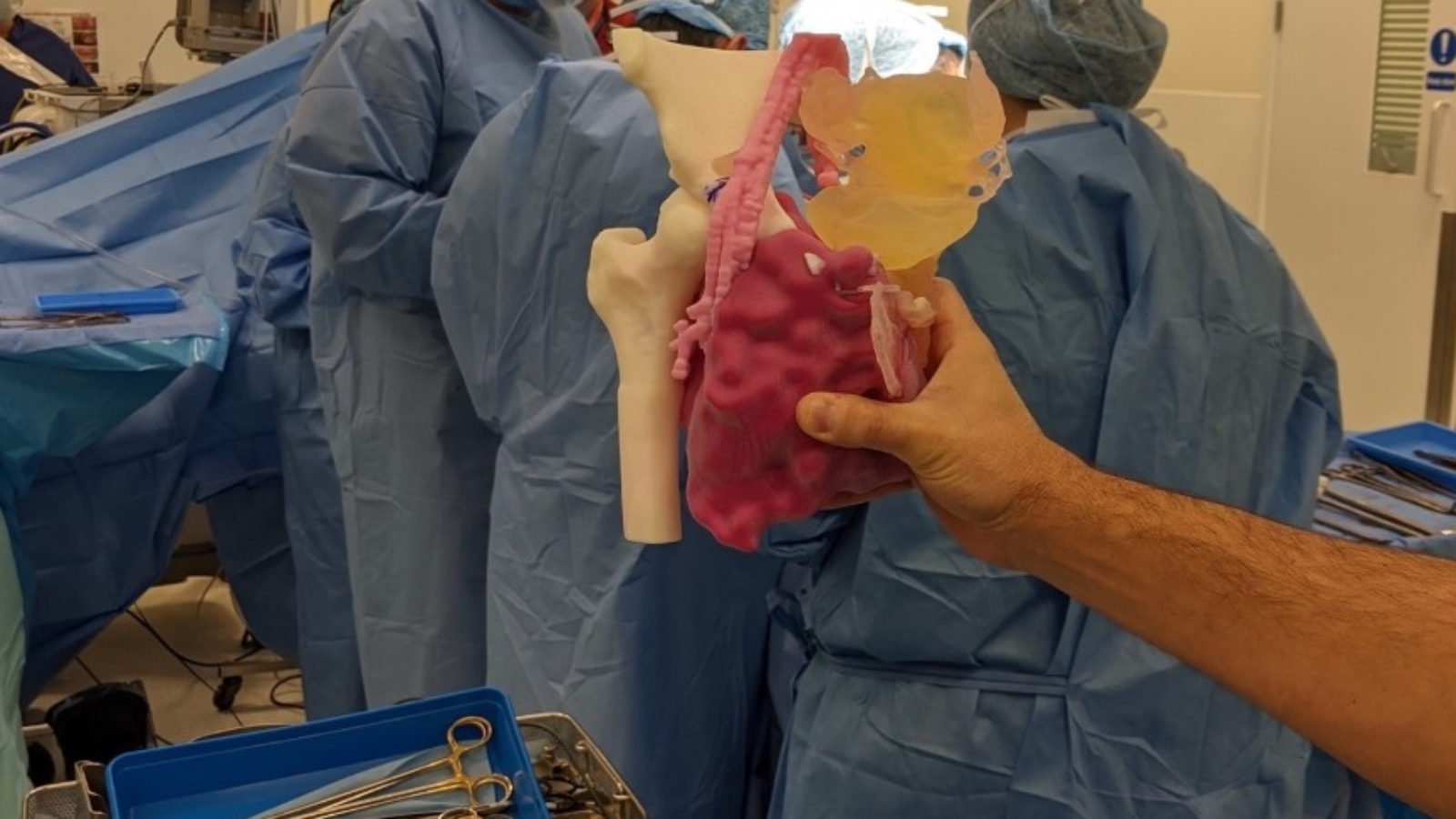
Initially established in 2013 as a humanitarian aid group providing prosthetics to African amputees, 3D LifePrints has since expanded, setting up four ‘innovation hubs’ across the UK. At each of these bases, the company offers to 3D print highly-accurate surgical models, which serve to help patients better understand their condition, as well as providing surgeons with a valuable pre-operative training tool.
Thanks to the FDM, SLS, SLA and PolyJet technologies housed at these hubs, 3D LifePrints says that host institutions can “enjoy all of the benefits of an ‘in-house’ service,” without having to spend big on new machines. In the past, using the firm’s install base, surgeons have been able to create cardiac models to reduce the risk of complications, as well as making custom face masks for burn victims.
Through successive funding rounds, the company has also sought to continually expand on its operations over the last three years, securing £500,000 worth of investment in January 2018. Since then, 3D LifePrints has managed to raise a further £1.2 million as a means of growing the adoption of its hub-based model, and it’s thought that the firm’s ISO certification will further this strategy moving forwards.

Medical 3D printing QA
3D LifePrints’ newly-announced ISO certification covers the “design and manufacture of sterilisable devices within a controlled environment” at its embedded 3D printing hubs. The award confirms that the firm is in compliance with an internationally-recognized set of guidelines, which are designed to ensure the development, production and shipping of only the highest-quality clinical products.
According to the International Standards Organization (ISO) itself, ISO 13485:2016 refers to QMS standards which demonstrate that medical devices “consistently meet customer and applicable regulatory requirements.” What’s more, to gain certification, firms must also prove that the products provided by their “associated services,” and “suppliers or external parties” meet these stringent guidelines as well.
Manufacturing on Demand
Now that the processes behind its Embedmed QMS have been certified, 3D LifePrint says that it’s able to “expand its industry-leading point-of-care model.” At present, the company’s operating model sees it deliver products-as-a-service from hubs embedded in host institutions such as universities, clinical practises and hospitals.
Already, the firm is working with the UK-based Alder Hey Children’s Hospital, Nuffield Orthopaedic Centre, Wrightington Hospital and Leeds General Infirmary, and having proven the quality control that goes into the on-demand devices produced there, it now anticipates further “market opportunities” for its 3D printing business ahead.
Surgical models ‘as-a-service’
Using 3D printing, it’s now possible to produce patient-specific anatomical models with high precision, which offer surgeons a better preoperative insight than that provided by traditional 2D imaging. At the UK-based Queen Elizabeth Hospital, for instance, surgeons have found that installing a Stratasys Objet 3D Printer has saved them 3-4 hours per operation.
Similarly, much like LIfePrint’s model-as-a-service business, Fast Radius and Axial3D launched a ‘DICOM-to-print’ service for surgeons and hospitals in North America last year. Working in tandem, the companies aim to provide clinicians with access to improved surgical planning via micro-millimeter accurate anatomical models with lead times of less than 48 hours.
Elsewhere, 3D printer manufacturer 3D Systems has iteratively built on the capabilities of its virtual surgical planning platform, which was cleared by the FDA in September 2019. The service effectively allows surgeons to turn CT scans of patients into detailed 3D images, which in turn, can be used as a means of planning highly-complex procedures.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment