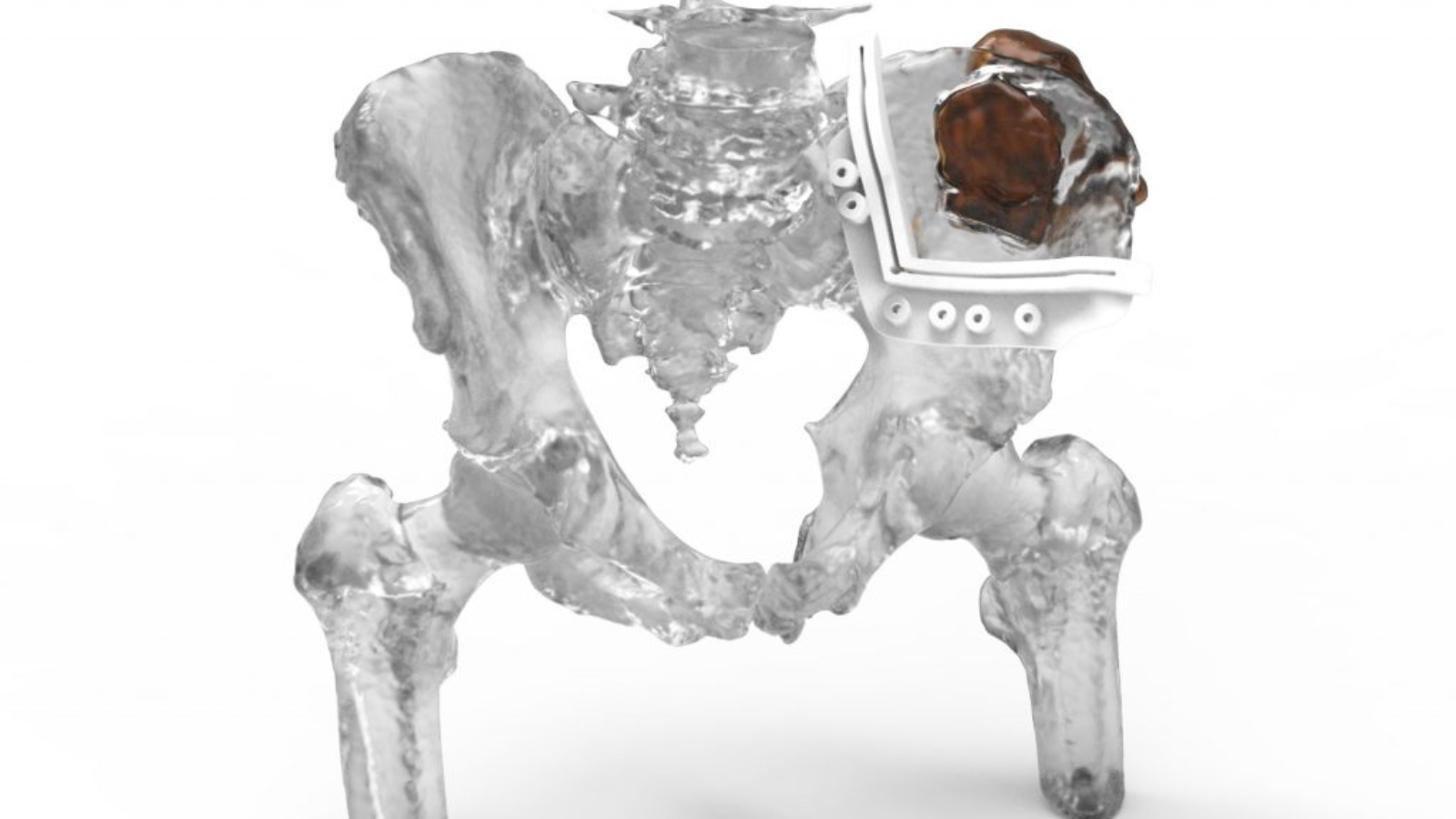
US-based 3D printer manufacturer 3D Systems has received U.S. Food and Drug Administration 510(k) clearance broadening the approved use of its VSP Orthopedics virtual surgical planning and patient-specific instrumentation platform. The clearance now covers skeletally mature adolescents with normal bone structure, in addition to adult patients. This change removes previous regulatory constraints that limited use in adolescent cases and formally brings these procedures within the platform’s labeled indications.
“This regulatory clearance removes a significant friction point for adoption in the pediatric/adolescent orthopedic oncology segment. Surgeons at leading centers have been using off-label or compassionate use solutions for years; this decision immediately converts those cases into routine clinical practice and opens the U.S. adolescent bone sarcoma and deformity market to our platform. We are thrilled to now offer these solutions to an expanded and underserved patient population,” said Ben Johnson, senior vice president of medical technology at 3D Systems.

Clinical and Market Implications
With this approval, hospitals and surgeons no longer need to rely on compassionate-use pathways or individual institutional review board approvals for eligible adolescent patients, reducing administrative burden and enabling more consistent clinical workflows.
The expanded indication addresses a segment that includes rare but complex orthopedic oncology cases, such as osteosarcoma and Ewing sarcoma in patients under 20, as well as additional primary bone cancer cases in young adults. It also applies to a broader range of reconstructive and corrective procedures, including lower-limb osteotomies related to congenital, developmental, or trauma-induced deformities in adolescents.
These procedures are already reimbursed under existing DRG and CPT codes, meaning no reimbursement changes are required to support adoption.

Financial Performance and Competitive Position
VSP Orthopedics is built on a clearly defined, repeatable revenue model that links each surgical case to multiple income streams, including fees for virtual surgical planning as well as sales of patient-specific, 3D printed anatomical models and single-use surgical guides produced on 3D Systems’ additive manufacturing platforms. This structure directly supports the medical technology segment’s sustained double-digit average annual growth and delivers gross margins that are meaningfully accretive compared with the company’s broader operations.
Manufacturing on Demand
At the same time, the expanded indication reinforces 3D Systems’ competitive position. The company is currently the only provider with FDA-cleared virtual surgical planning solutions that span craniomaxillofacial and orthopedic applications, now including eligible adolescent patients.

Recent FDA Approvals in 3D Printing Medical Innovations
The expanded FDA clearance for 3D Systems’ VSP Orthopedics platform aligns with a broader pattern of regulatory progress in medical 3D printing.
In June, U.S.-based 3D printer manufacturer 3D Systems, in partnership with French MedTech firm TISSIUM, has secured FDA approval for a bioabsorbable, 3D printed device designed to treat peripheral nerve damage. This approval validates the polymer’s clinical effectiveness and paves the way for its use across a wide range of medical treatments.
In March 2025, Triastek, a Chinese pharmaceutical company specializing in 3D printing, announced that its proprietary 3D printed non-vitamin K antagonist oral anticoagulant (NOAC), T20G, received Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration as of February 27, 2025. This regulatory milestone follows an earlier IND approval granted by China’s National Medical Products Administration (NMPA) in January 2024.
You might also like:
3D Printed Biodegradable Scaffolds Offer Potential for Improved Heart Bypass Grafts: “I’m really excited about translational research that breaks ground scientifically but also has the potential to improve people’s lives,” Yonghui Ding, Assistant Professor, Department of Biomedical Engineering added. “Many people need bypass surgery, and our research could result in better grafts that lead to better health outcomes for patients.”
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paloma Duran

Leave A Comment