U.S.-based 3D printer manufacturer 3D Systems, in partnership with French MedTech firm TISSIUM, has secured FDA approval for a bioabsorbable, 3D printed device designed to treat peripheral nerve damage. This approval validates the polymer’s clinical effectiveness and paves the way for its use across a wide range of medical treatments.

Breakthrough in Peripheral Nerve Repair
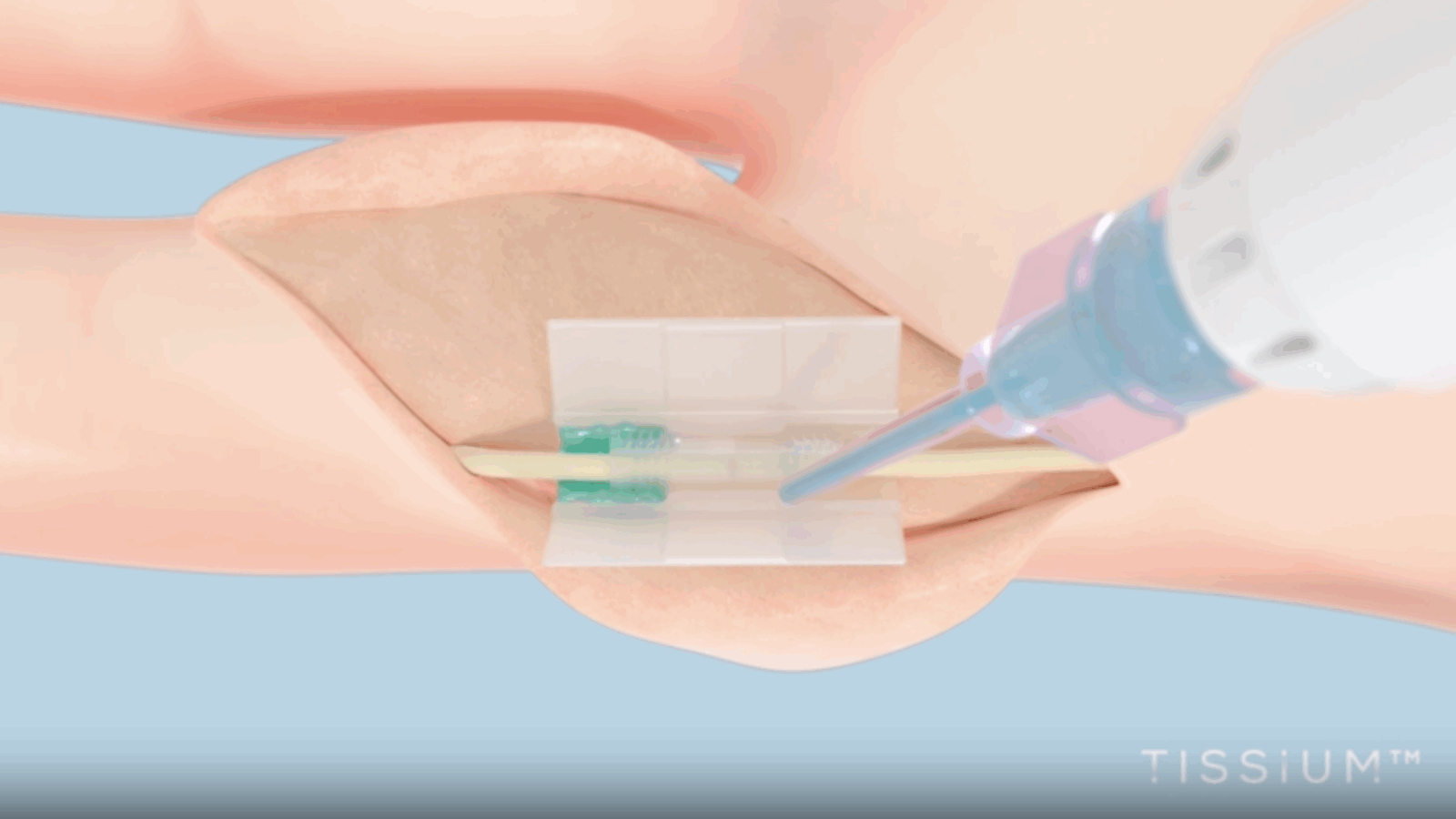
For several years, 3D Systems and TISSIUM have collaborated to develop a tailored 3D printing approach for repairing damaged peripheral nerves. Combining TISSIUM’s proprietary biomorphic polymers with 3D Systems’ bioprinting technology, they created COAPTIUM CONNECT with TISSIUM Light — a fully bioabsorbable, photopolymer-based medical device. This device is the first of its kind: a sutureless, atraumatic solution that facilitates peripheral nerve repair.
3D Systems stated that thanks to the polymer’s unique properties, the device enables the creation of high-resolution, elastomeric, biodegradable implants that are unmatched in the industry.
“This is a significant advancement in patient care,” said Scott Turner, vice president of advanced systems at 3D Systems. “It has been tremendously rewarding to work alongside the talented team at TISSIUM to design a complete 3D bioprinted solution that offers the potential for patients to recover from peripheral nerve damage.”

3D Systems’ Broader Regenerative Medicine Vision
This FDA approval complements 3D Systems’ extensive work in regenerative medicine. Since 2017, the company has collaborated with United Therapeutics to create an unlimited supply of human lungs that do not require immunosuppression, enabling all patients with end-stage lung disease to receive transplants and live longer, healthier lives. Central to this work is 3D Systems’ Print to Perfusion process, which produces high-resolution scaffolds capable of being seeded with living cells to form functional tissue. By combining advanced bioprinting techniques with biocompatible materials and multiple cell types—including patient-derived cells—the company is delivering personalized, living tissues.
“3D Systems is making a profound impact not only on how healthcare is delivered, but on the quality of patients’ lives, and continues to solidify what I believe is an unparalleled role we play in advancing medicine with additive manufacturing applications. This latest accomplishment by TISSIUM, enabled by our unique 3D printing technology, is one more example of how 3D Systems is transforming patient care for a better future,” said Dr. Jeffrey Graves, president & CEO, 3D Systems.
Manufacturing on Demand

Looking ahead, 3D Systems aims to capitalize on the global 3D bioprinting market, which is projected to surpass $2.4 billion by 2029. The company has supported more than 150,000 patient-specific surgical procedures and manufactured over two million implants and instruments from FDA-registered, ISO 13485-certified facilities in both the U.S. and Europe.
Recent FDA Approvals in 3D Printing Medical Innovations
In addition to the FDA approval of the 3D printed nerve repair device by 3D Systems and TISSIUM, several other projects have recently received FDA clearance.
In March 2025, Triastek, a Chinese pharmaceutical company specializing in 3D printing, announced that its proprietary 3D printed non-vitamin K antagonist oral anticoagulant (NOAC), T20G, received Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration as of February 27, 2025. This regulatory milestone follows an earlier IND approval granted by China’s National Medical Products Administration (NMPA) in January 2024.
In 2024, 3D Systems also received 510(k) clearance from the Food and Drug Administration (FDA) for its multi-material, 3D printed denture offering. Launched last February, the jetted, monolithic (one-piece) dentures are being billed as a “first-to-market” product that offers unparalleled break resistance and optimal aesthetics. The novelty of this offering lies in the integration of 3D Systems’ NextDent Jet Denture Teeth and NextDent Jet Denture Base materials into a single denture solution. It also leverages the company’s MultiJet Printing (<a href="https://facfox.com/service/polyjet-mjp-3d-printing-services” target=”_blank” >MJP) technology, software and services.
You might also like:
Dimension Ortho Partners with Rothman Orthopaedics to Advance Personalized Fracture Care: “Our goal is to provide more streamlined and patient-centric, comfortable recovery,” said Michael Rivlin, MD, President and Co-Founder of Dimension Ortho. “Dimension Ortho’s partnership with Rothman Orthopaedics will allow thousands of patients to experience minimal disruption to daily activity and accelerated recovery from both acute and chronic injuries.”
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paloma Duran

Leave A Comment