US 3D printer manufacturer 3D Systems has entered into a partnership with artificial intelligence (AI) medical firm Enhatch to make the design and delivery of patient-specific medical devices more efficient.
Through the partnership, the companies will combine their respective expertise and technologies to create an optimized, automated, and scalable workflow for fabricating medical devices.
“Our partnership with Enhatch will enable us to deliver the healthcare industry’s most comprehensive approach to additive manufacturing,” said Menno Ellis, Executive Vice President, Healthcare Solutions, at 3D Systems. “Integrating these technologies and capabilities into 3D Systems’ surgical planning solutions will make processes more efficient, trackable, and cost-effective.
“This is another step in our ongoing commitment to innovation that helps our customers remain at the forefront of medical device development and healthcare delivery.”
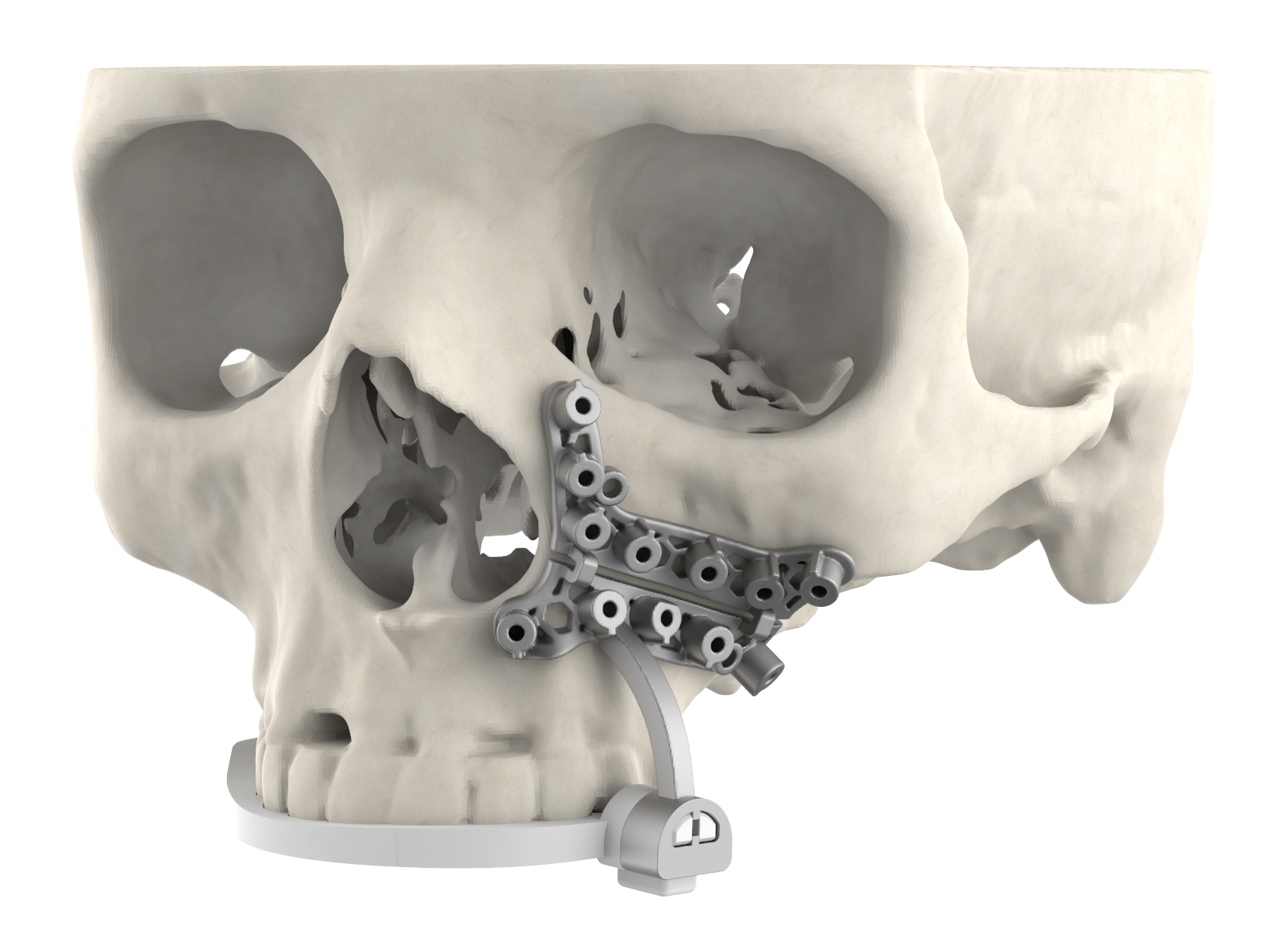 The new surgical guide’s dental registration is designed to provide surgeons with greater site visibility. Image via 3D Systems.
The new surgical guide’s dental registration is designed to provide surgeons with greater site visibility. Image via 3D Systems.
3D Systems’ VSP medical portfolio
3D Systems’ Virtual Surgical Planning (VSP) is central to the firm’s clinical offering, combining imaging, simulation, and 3D printing to create a fully-fledged surgical plan. The company carried out a ‘strategic resizing’ last year to focus on its core medical and industrial verticals, and has expanded its medical portfolio after achieving a ‘breakthrough’ in 3D bioprinting with its Print to Perfusion platform.
Alongside its growing range of FDA-approved implants, 3D Systems’ VSP anatomical offerings have been deployed in more than 140,000 patient cases to date. The firm’s VSP Orthopaedics platform gained 510(k) clearance from the FDA in 2019, and the following year 510(k) clearance was given to 3D print maxillofacial surgical guides and the firm’s Vantage Ankle PSI surgical instrument.
In April last year, the company added a new hybrid maxillofacial surgical guide to its VSP portfolio, which reportedly combines the strength of titanium with the softness of nylon.
Accelerating 3D printed medical device delivery
Enhatch is building an AI-powered open portfolio of technologies designed to personalize and accelerate the entire surgical workflow, called the Intelligent Surgery Ecosystem. The technologies are engineered to streamline and scale the design and delivery of patient-specific medical devices by automating the process.
Manufacturing on Demand
The new partnership with 3D Systems will see these capabilities integrated with the printer manufacturer’s workflow for patient-specific medical offerings, which include advanced software, treatment planning services, custom 3D printed implants, and surgical instrumentation design.
By combining Enhatch’s AI-driven surgical technologies with 3D System’s VSP workflow, the companies hope to reduce procedure times and improve surgical outcomes for craniomaxillofacial and orthopedic medical specialties. The combination will ultimately create an automated and scalable medical device 3D printing workflow to meet the ever-growing demand for personalized medical devices.
“Enhatch is proud to welcome 3D Systems to the Intelligent Surgery Ecosystem,” said Peter Verrilo, Co-founder and CEO of Enhatch. “Healthcare ecosystems have tremendous potential to disrupt and reshape the entire industry, leading to improved patient outcomes, faster, more accurate, and safer procedures.
“Enhatch and 3D Systems have a shared goal of bringing surgeons and industry together with the best leading-edge technologies available.”

Regulating 3D printed medical devices
In the past, there have been calls for clearer regulation of 3D printed medical devices and greater clarity and cooperation between manufacturers and regulators. At the tail end of last year, the FDA issued a public call for feedback on the regulation of 3D printed medical devices for a framework it is designing to ensure the quality of such devices.
Alongside 3D Systems’ VSP offerings, numerous other 3D printed medical applications have received FDA clearance, such as drug-loaded tablets, hearing aids, implants that support bone growth, and proprietary resins for dental prosthetics. The FDA has also green-lit full-scale facilities to offer 3D printing services to medical manufacturers at scale.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Hayley Everett

Leave A Comment