Over the last decade, 3D bioprinting has come on leaps and bounds, with significant advances being made in the development of viable, patient-specific soft tissues.
Using microscale skin models, scientists such as those at the University of Stuttgart, are now working towards trialling the efficacy of cancer drugs and potentially making animal testing obsolete, while commercial firms like CELLINK have committed to the same cause, with its CEO Erik Gatenholm recently reiterating its aim of “reducing, and in some cases eliminating, animal testing by providing alternative models.”
“I think this field has made major progress in the last five years, and the 3D bioprinting of anatomically-relevant structures is going well,” agrees Ibrahim Ozbolat, an Associate Professor at Penn State and leading bioprinting researcher. “Multiple companies are trying to commercialize their support structures for bioprinting purposes, so there’s been quite a bit of progress made in that direction.”
By contrast, other academics such as Juan Carlos Marvizon of the Brain Research Institute, say that fabricated human tissues are highly experimental, and remain decades away from human drug testing applications, if they’ll ever be ready at all.
“The expected shift from animal testing models to in-vitro systems is just not happening,” said Marvizon. “The problem is that what matters is the interaction between tissues, for example, between the CNS and the immune system. Unless you can 3D print the whole body, you will not be able to predict what a drug will do to a patient.”
To assess the claims on either side of this bioprinting debate, as well as the current state of the field, 3D Printing Industry spoke to experts at the Brain Research Institute, Penn State University, CELLINK, Fluicell, CLECELL, Mimix Biotherapeutics, 3D Bioprinting Solutions, UpNano and Aspect Biosystems, who have each offered their guidance on the technology’s pitfalls and potential moving forwards.

Microfluidic bioprinting’s potential
In general, 3D bioprinting research can be broken down into projects conducted using extrusion, inkjet and SLA, as well as pressure and laser-assisted technologies, but within these broader approaches, microfluidics advances, in particular, are increasingly enabling human tissues to be produced with extreme precision and at a microscale.
Since launching its Biopixlar platform in 2019, Fluicell has become one of the driving forces behind this boom in microfluidic bioprinting R&D. Thanks to the precision of its proprietary system, the firm has already been able to produce highly-complex neural models, and it sees great potential for the technology in creating soft tissues with future clinical drug screening applications.
“The Biopixlar’s ability to print cells at a very high resolution enables researchers to achieve more relevant research models,” explained Jonas Hannestad, CMCO of Fluicell. “The greatest potential for using bioprinting to replace the use of animals for research, lies in using bioprinted models to acquire better knowledge about drug effects earlier in the development process.”
Elsewhere, Canadian firm Aspect Biosystems has also adopted a microfluidic 3D bioprinting approach, but while placing an emphasis on creating “off-the-shelf” therapeutics rather than drug testing tools, that address the “scaling difficulties” which the company believes to have previously prevented the technology’s application as an alternative testing model.
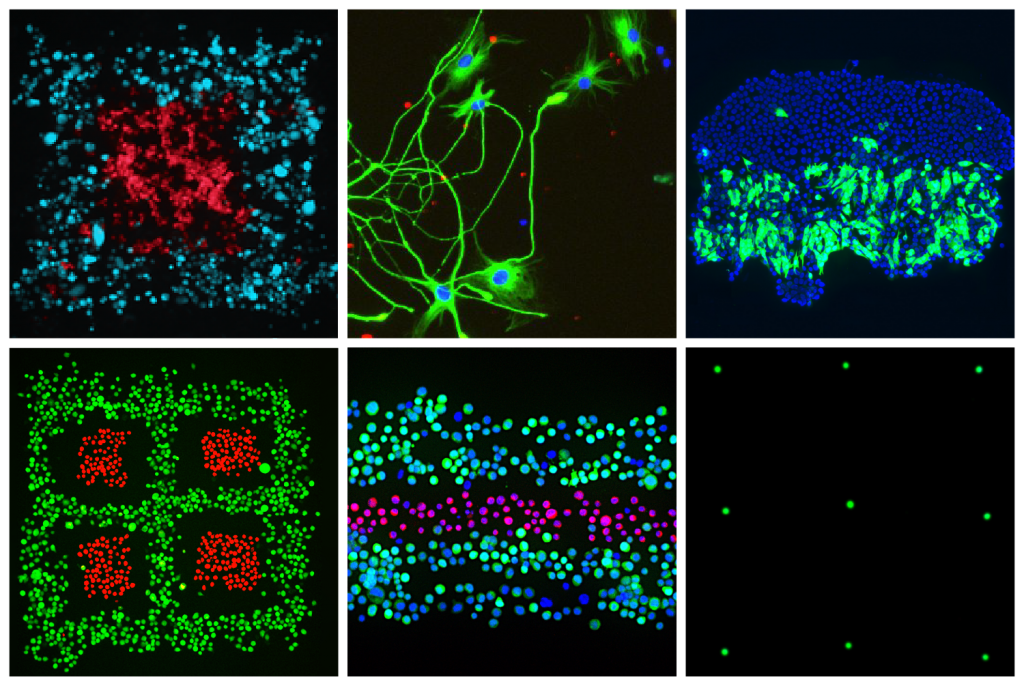
“While we are not internally-focused on applying our technology towards drug screening, we do believe that it could provide more predictive data in the drug development process,” said Natalie Korenic, Senior Manager, Marketing & Communications at Aspect Biosystems. “Our current programs are focused on diseases of the pancreas and liver, as they are currently in animal testing and being driven towards the clinic.”
The limitations of artificial tissues
Although microfluidic 3D bioprinting has shown great patterning potential, maintaining cell viability within larger structures remains a challenge to their wider adoption. To combat this, firms such as CELLINK say that cell-based additive manufactured models now need to make the transition from the lab into clinical studies, before they can be considered a viable substitute for animals within drug testing.
“3D bioprinted tissues can give faster results compared to animal models, allowing users to more rapidly iterate on experiments,” explained Ariel Kramer, CCO of CELLINK. “The current limitation is the need to produce constructs at scale, but also those that mimic the in-vivo environment of the lab. CELLINK’s technologies such as the BIO MDX [3D bioprinter] are looking to address these issues.”
Mimix Biotherapeutics, meanwhile, is taking the approach that speed and simplicity are the key ingredients to viable bioprinted tissues. Building on these core principles, the firm has iteratively developed its cymatiX acoustic bioprinting platform, which uses sound-wave energy to manipulate cell clusters without causing them to undergo shear stress.
According to the company, its technology is capable of producing soft tissues at scale, which address applications ranging from drug R&D and regenerative medicine to vegan-friendly meats. However, even though Mimix’s systems have now made it through to preclinical trials, it concedes that product certification remains one of several challenges preventing bioprinting from reaching commercial fruition.
“The biofabrication toolbox is facing challenges such as in-vivo biological relevance, cell quantities, limited accessibility, micro/macro vascularization, and also regulatory and societal challenges,” said Marc Thurner CEO of Mimix Biotherapeutics. “Today, we feel confident that acoustic biofabrication will bring solutions to overcome some of the current hurdles related to vascularization and in-vivo relevance.”
“CymatiX is simple, fast and reproducible. In a few seconds you can build large scale cellular layers with controlled architectures to mimic the extracellular environment.”
Interestingly, UpNano has also entered the 3D bioprinting arena with the recent launch of its NanoOne Bio system, but says that “it doesn’t aim to print holistic tissue structures.” Instead, the firm is focused on enabling the creation of cell-culturing microstructures, which could help reduce the number of animal experiments behind clinical trials in the longer term.
In fact, speaking to 3D Printing Industry, UpNano has said that its machine is “already being used by customers in the biological environment and has proven itself,” and while it also sees printing material certification as one of the technology’s barriers to entry, it remains “convinced that 3D printing will establish itself in medical research.”

Disease modeling in-action
Although research into scalable 3D bioprinting remains ongoing, the technology has now reached a point where it’s increasingly finding experimental disease modelling applications. Korean firm CLECELL, for instance, has leveraged its U-FABTM microinjection platform to 3D print a lifelike epithelium model that has the potential to model respiratory infections.
Manufacturing on Demand
“We have established a protocol for producing a 3D respiratory epithelium model for the study of diseases such as COVID-19,” said Su Yun Chae, CMO of CLECELL. “Currently, we are also developing breast cancer models including a mammary duct, glioblastoma model, malignant brain tumor as well as a skin cancer model.”
“If 3D bioprinted cancer tissue is produced using the patient’s cancer tissue, their treatment is expected to improve greatly because the ‘optimal anticancer drug selection and drug effect’ can be accurately predicted.”
Likewise, the biotechnical research lab 3D Bioprinting Solutions has made significant progress when it comes to creating implantable human cells. Using their ORGAN.AUT system onboard the ISS, the lab’s scientists have already managed to fabricate bone tissue, and with further validation, scaling and standardization, they believe that it could become a vital disease modelling tool as well.
“During the pandemic, testing different drugs on 2D tissues did not show a good efficacy,” said Yusef Khesuani, Co-founder of 3D Bioprinting Solutions. “To examine anti-COVID-19 drugs, scientists have had to use transgenic animals, and that was a big problem not only to many labs, but also to many countries.”
“We need to be ready for such situations. 3D bioprinted constructs could become good solutions for such unpredictable situations,” he added. “I think that 3D bioprinted constructs will most probably act as a substitute for 2D tissues in drug testing in the near future.”

Bioprinting: the experts’ verdicts
Much like those seeking to commercialize 3D bioprinting, there’s considerable disagreement among researchers when it comes the technology’s potential in producing soft tissue animal testing substitutes. As a specialist in neurophysiology, Marvizon is unequivocal in his doubts about 3D bioprinting’s ability to accurately replicate the most intricate natural structures found inside the human body.
“In my field of pain research, I haven’t seen any papers use 3D printing,” said Marvizon. “In chronic pain, relevant events happen in the Central Nervous System (CNS), mostly in the dorsal horn of the spinal cord. It would be extremely difficult and costly to 3D print the enormously complex structure of the dorsal horn.”
“Microglia undergo drastic changes in a matter of minutes. How are you going to 3D print that?”
Marvizon is also skeptical about the technology’s drug development potential, saying that the “rules of biomedical research are changing” in a way that “developments in biotechnology and nanotechnology will make the whole discussion irrelevant long before 3D printing is ready for even a modest contribution to drug development.”
Ozbolat, on the other hand, argues that 3D bioprinting could indeed become applicable within future drug testing evaluations, but “it depends on the tissue.”
“In major organs, there’s a lot of interactions with other systems in the body,” explained Ozbolat. “If you’re building something like the gastrointestinal (GI) tract, it also interacts with the brain, and it’s really tough to create a model that has both the GI train and the brain. But, if it’s just skin that has minimal interactions with other organs, we don’t have a better representative.”
In conclusion, Ozbolat added that he agrees with the concerns of many bioprinting firms, in that “clinical translation has been one of the major issues until now.” In an attempt to align the goals of 3D bioprinting researchers with those of regulators, Ozbolat has therefore met with the FDA, and presented the technology’s latest intraoperative use cases.
“I’d like to get feedback from the FDA about the potential of the clinical translation of these techniques, I really want to see what they think about what we do,” added Ozbolat. “It’s not really about supporting us financially, it’s more about supporting our technology’s transfer [into clinical settings].”
How ready is 3D bioprinting?
3D bioprinting research may still be at an experimental stage, but there’s no doubt that new approaches are beginning to accelerate the technology’s development. For instance, Munich University of Applied Sciences researchers have developed a method of single-cell resolution printing, while Readily3D is working towards 3D bioprinting a human pancreas for testing future diabetes medications.
However, before such innovations can ever be deployed within clinical settings, many of the industry’s experts agree that issues surrounding tissue vascularization and certification need to be resolved, and the technology’s applications may ultimately prove limited when it comes to replicating the complex interactions that take place inside certain areas of the body.
In this light, 3D Systems’ continued Print to Perfusion program and its attempt to develop bioprinted transplantable organs, could prove to be a more viable technological application. Although printing fully-sized organs remains some way from reality, they can theoretically be programmed to prevent rejection from their hosts, potentially making them a more promising avenue of research than drug testing platforms.
“Perhaps the key is not to look at 3D printing as a tool for drug testing, but as a technology with utility on its own,” concluded Marvizon. “You have to work together with molecular biology. For example, you could 3D print an ACL instead of using one from a cadaver. Using DREADDs [manipulated proteins], you can stimulate its growth and facilitate its interaction with the immune system, avoiding tissue rejection.”
Join 3D Systems, Cellink, Lund University and 3D Printing Industry this Thursday for a free webinar to discuss the future of regenerative medicine, limited places available – register now.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy


Leave A Comment