Triastek, Inc., a company specializing in 3D printing of pharmaceuticals, entered into a collaboration with Eli Lilly and Company, a leading global pharmaceutical company, to leverage the advantages of MED 3D printing technology to enable precisely targeted and programmed release of drugs in specific regions of the GI tract.

According to the agreement, the project will focus on the targeted release of drugs in the intestine. Triastek will focus on two aspects: Firstly, conduct an in-depth study of excipient properties and process parameters to maintain drug stability throughout the formulation development and 3D printing process, as well as during drug release. Secondly, identify a unique three-dimensional structure dosage form design, that will permit the programmed release of drugs in specific parts of the intestine, with the goal of improving the bioavailability of orally administered drugs.
Manufacturing on Demand
Dr. Senping Cheng, founder and CEO of Triastek, commented: “The collaboration between Triastek and Lilly is a great example of applying MED technology for improving the oral delivery of drugs. We envision that the MED technology of Triastek can be used to solve the challenges in formulations leading to the development of clinically valuable products for our global partners.”
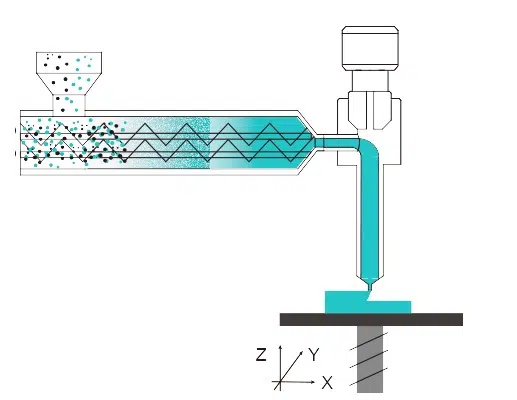
Triastek’s pioneering technology, which it refers to as MED (Melt Extrusion Deposition) 3D printing technology, has versatile applications in solid dosage form development and manufacturing. With the facilitation of this collaboration by Lilly China Innovation & Partnerships, Triastek will work with Lilly to explore novel solutions to the oral delivery of drugs.
Triastek is committed to promoting the application of 3D printing technology in the pharmaceutical field. Triastek’s 1st and 2nd products (T19 and T20) have received IND clearance from the U.S. Food and Drug Administration (FDA). The company also holds 158 patent applications related to 3D printing of pharmaceuticals with comprehensive patent coverage in the world. The company has also established collaborations with a number of multinational pharmaceutical companies, as well as domestic pharmaceutical companies to provide technical solutions for the development of challenging formulations.
* This article is reprinted from 3D Printing Media Network. If you are involved in infringement, please contact us to delete it.
Author: 3D Printing Media Network


Leave A Comment