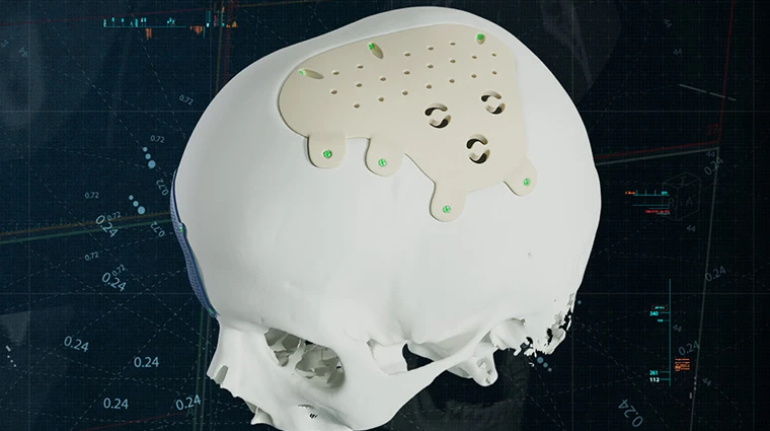
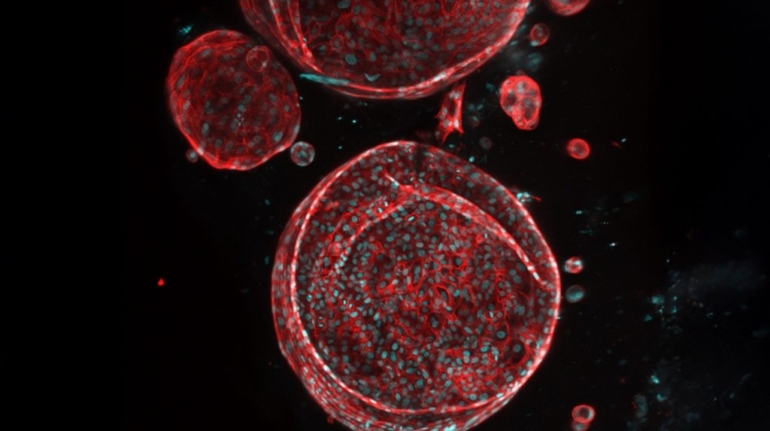
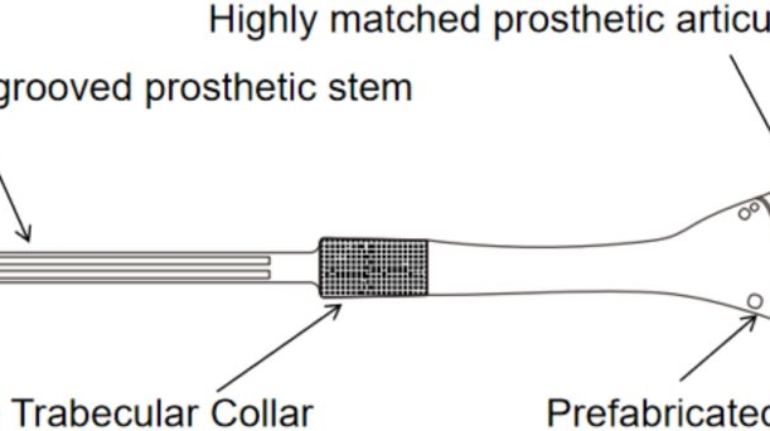
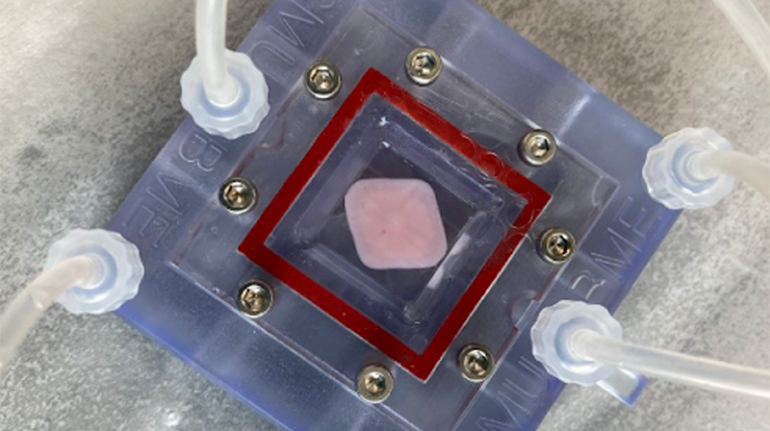
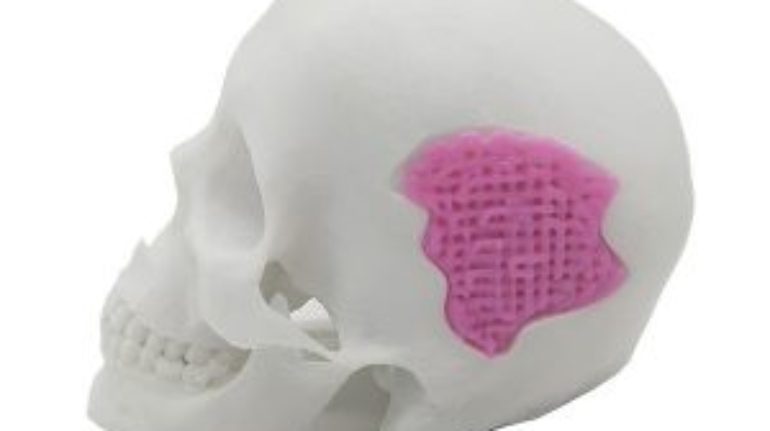
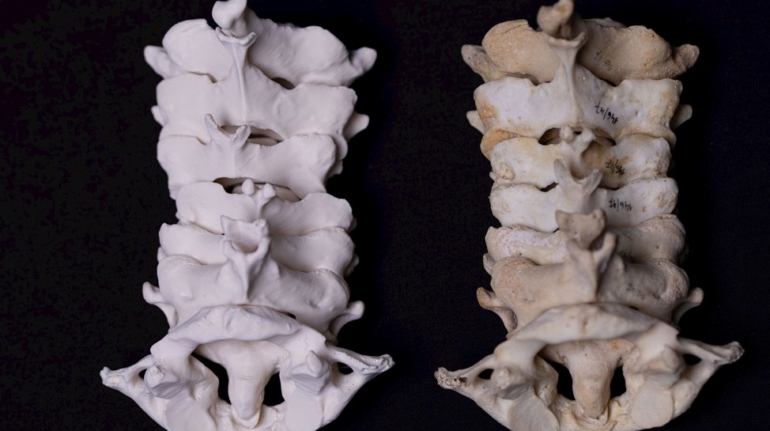
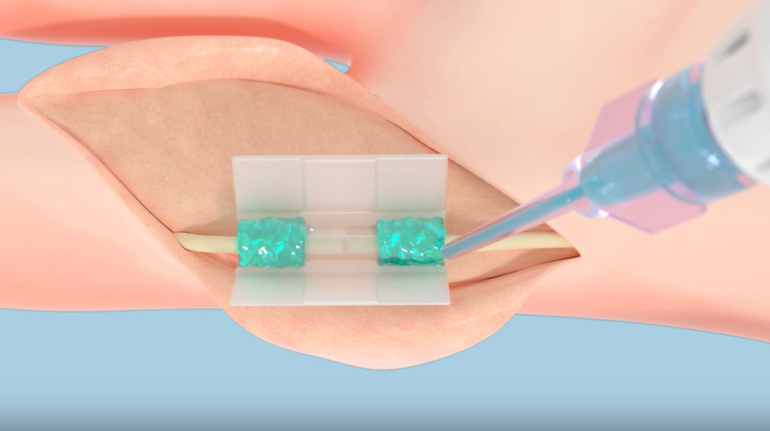
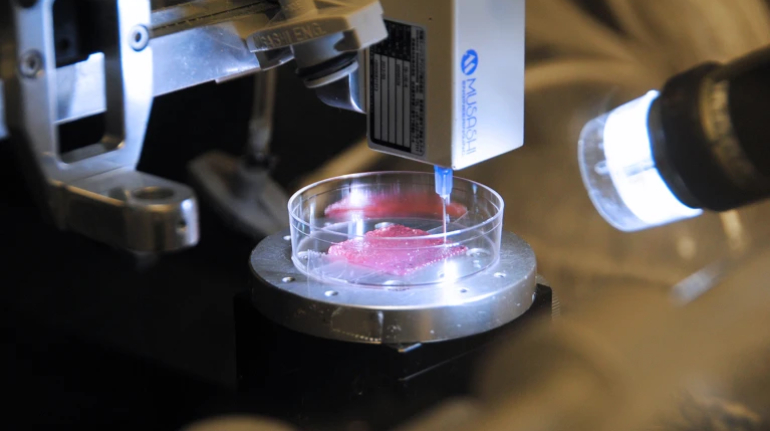
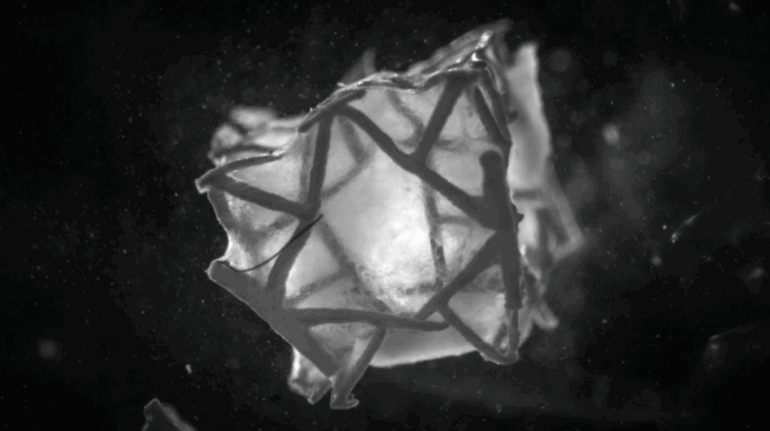
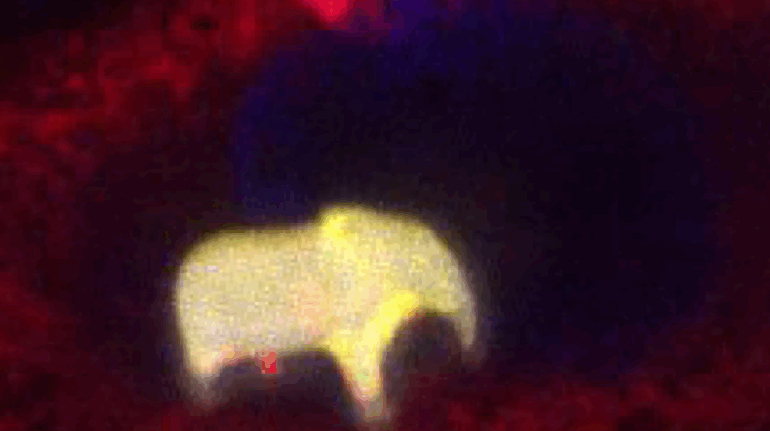
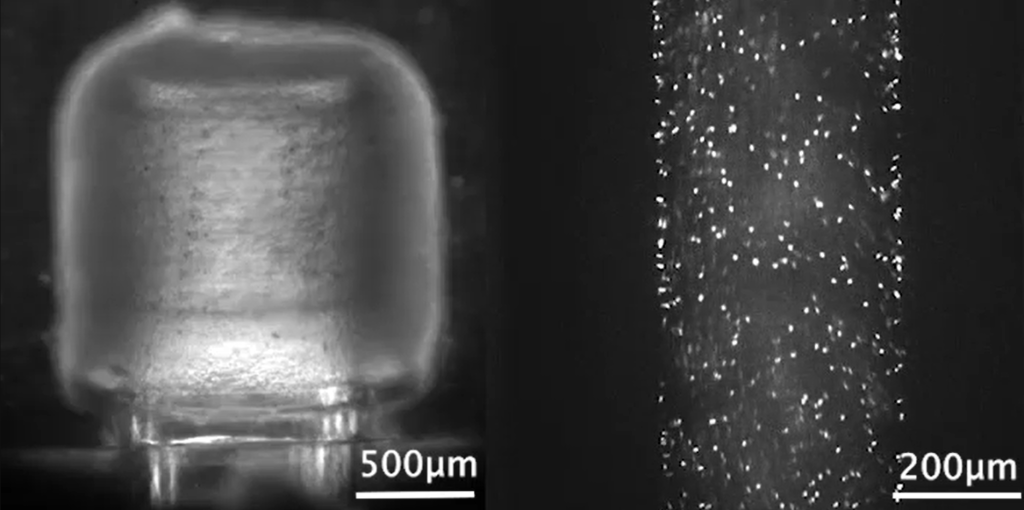
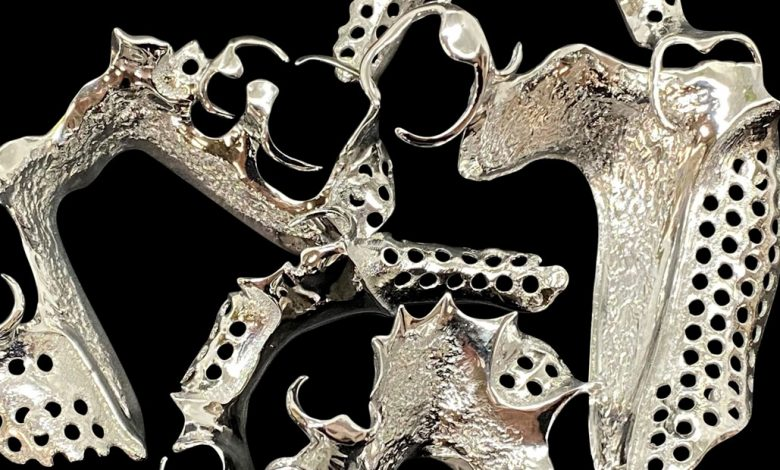
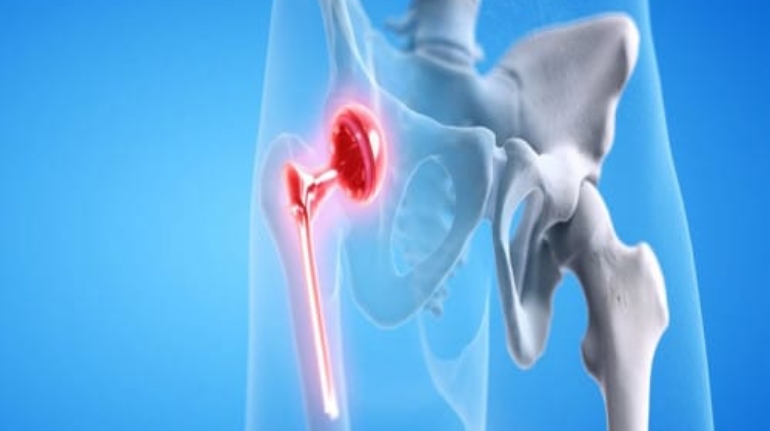
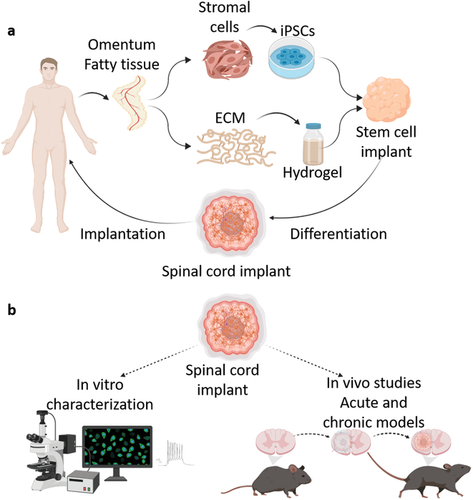
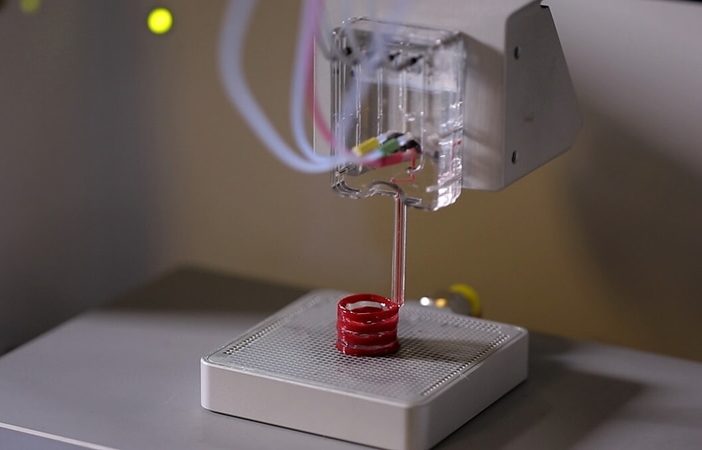
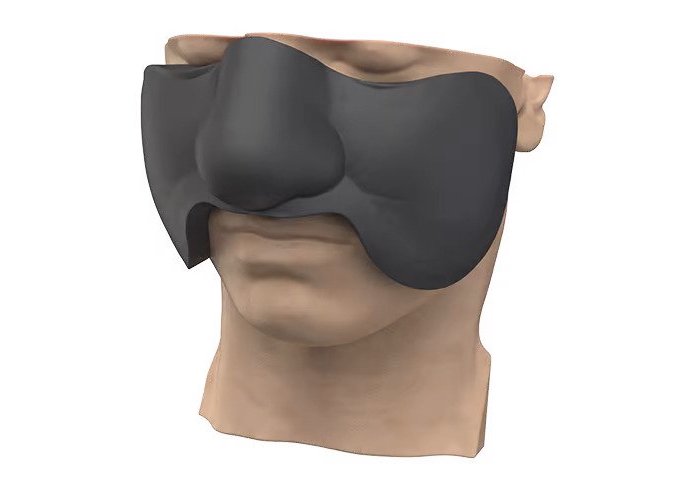
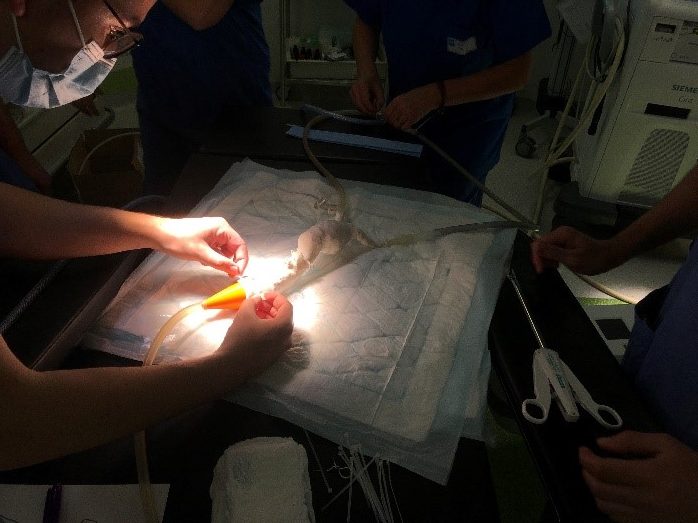
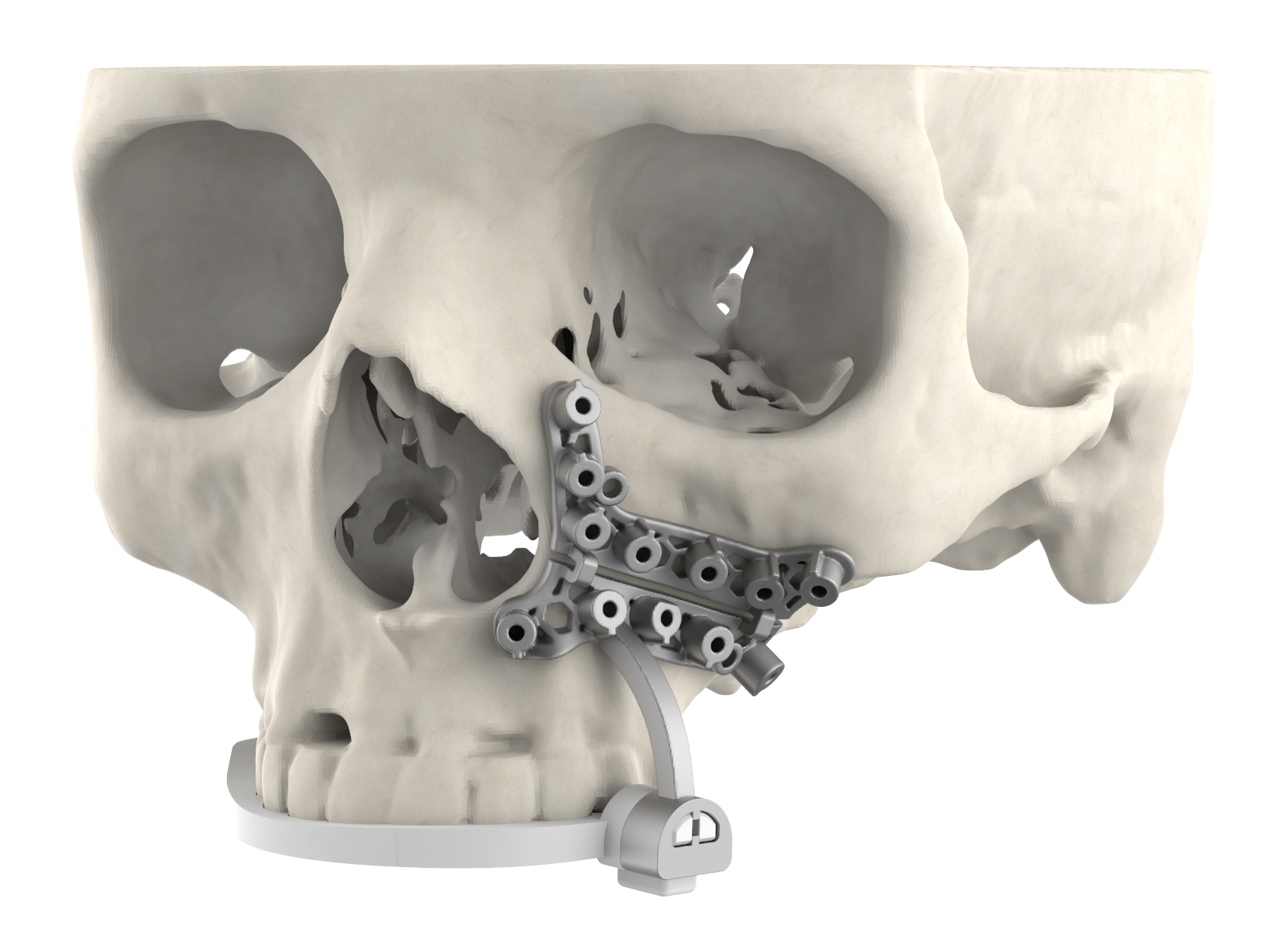
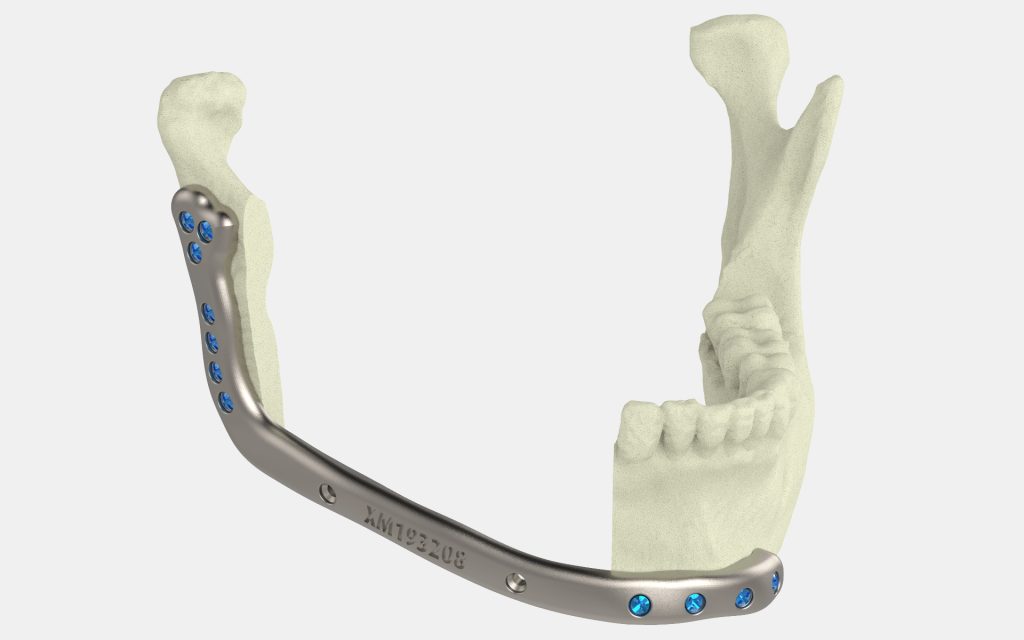
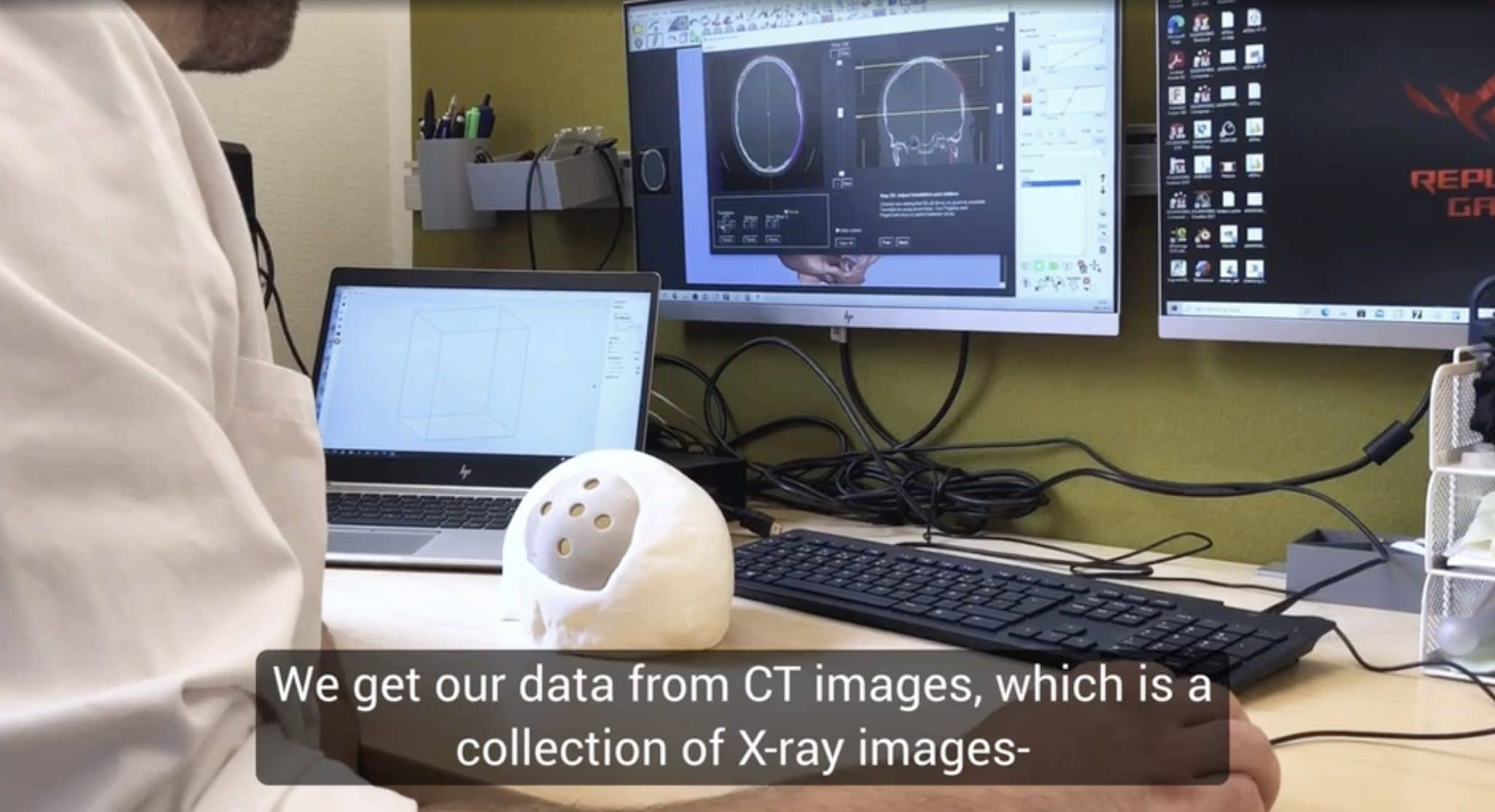
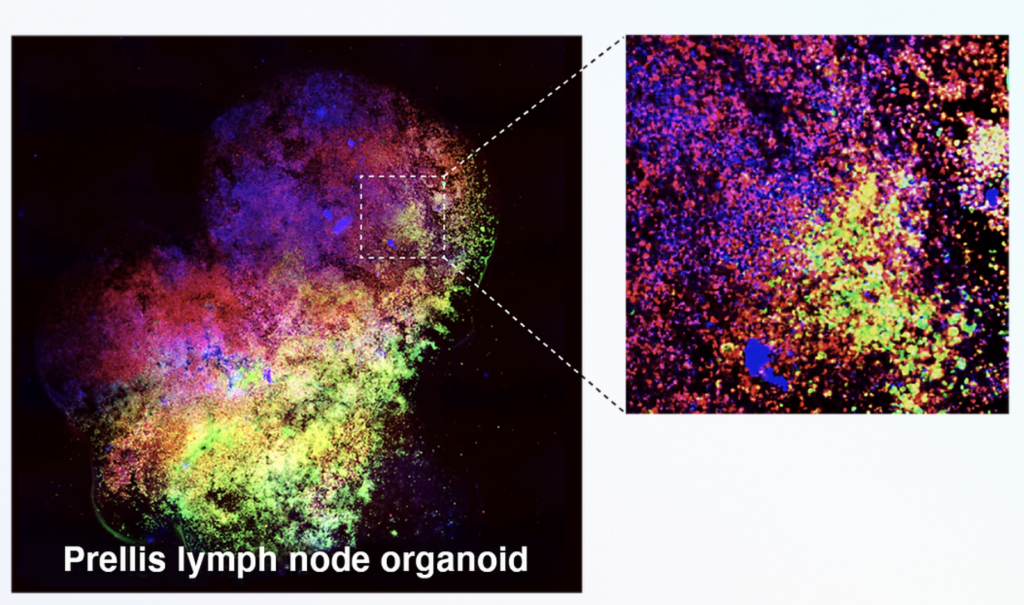
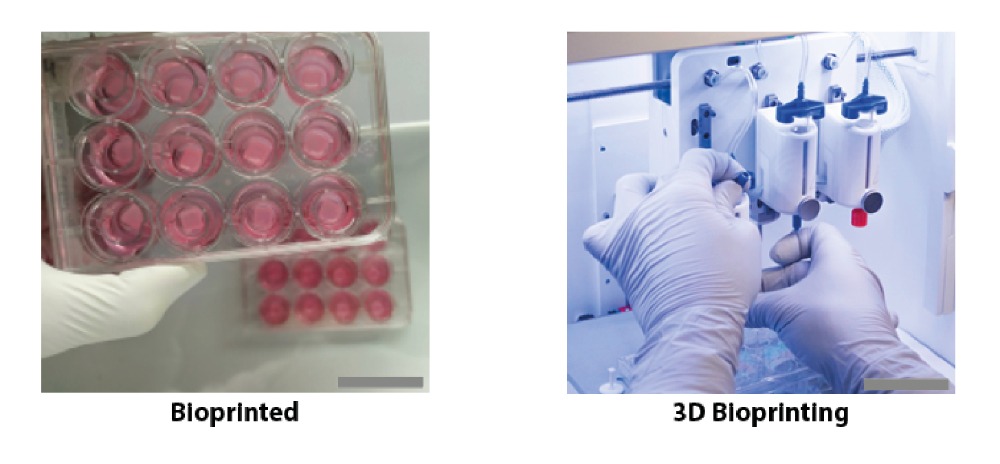
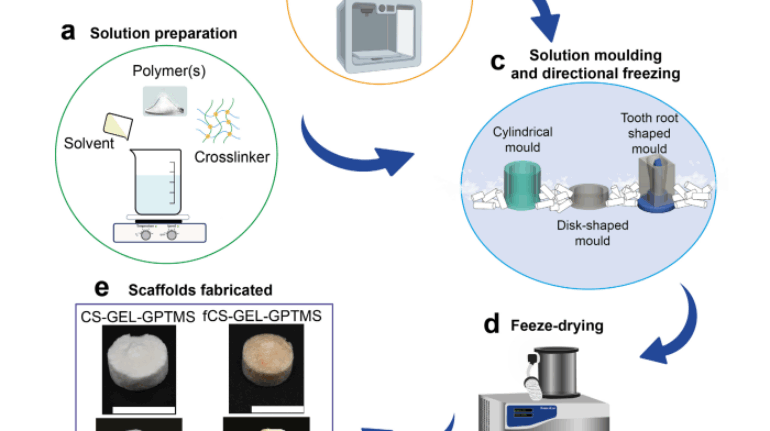
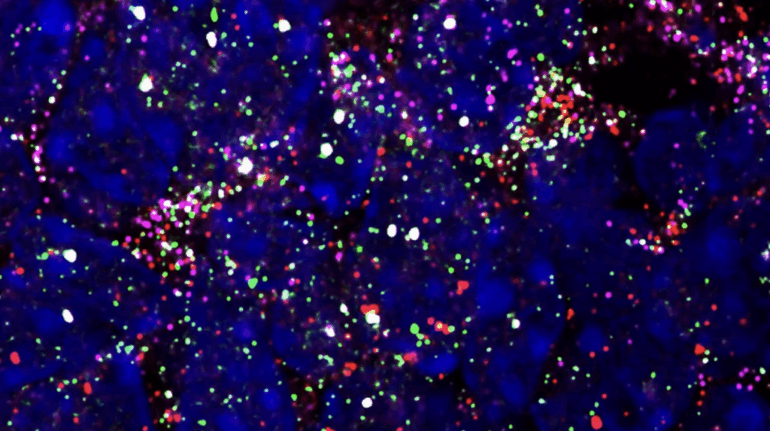
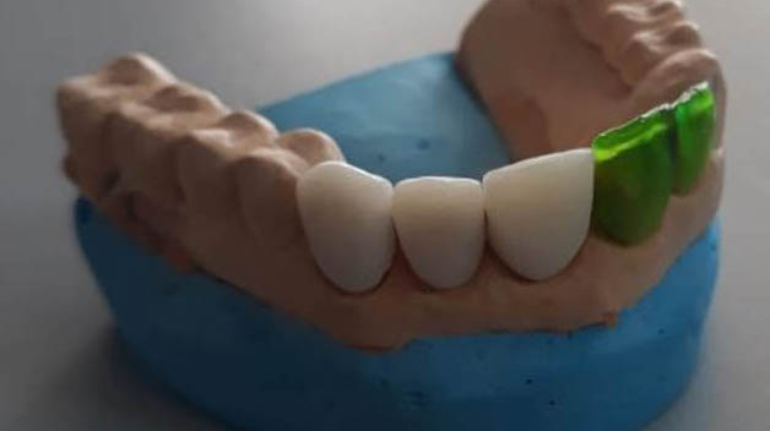
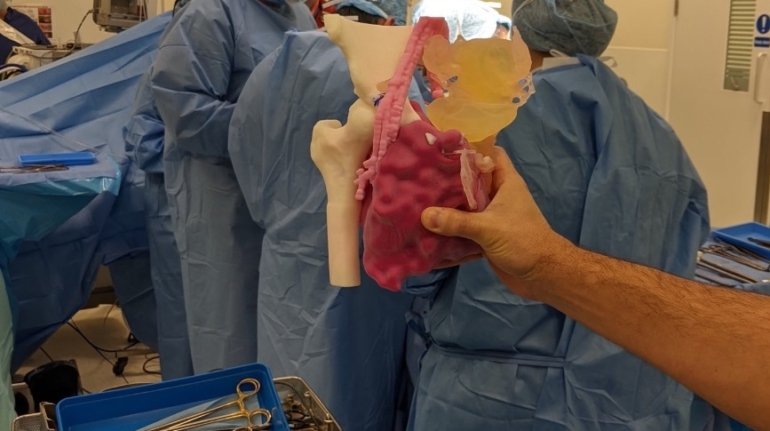
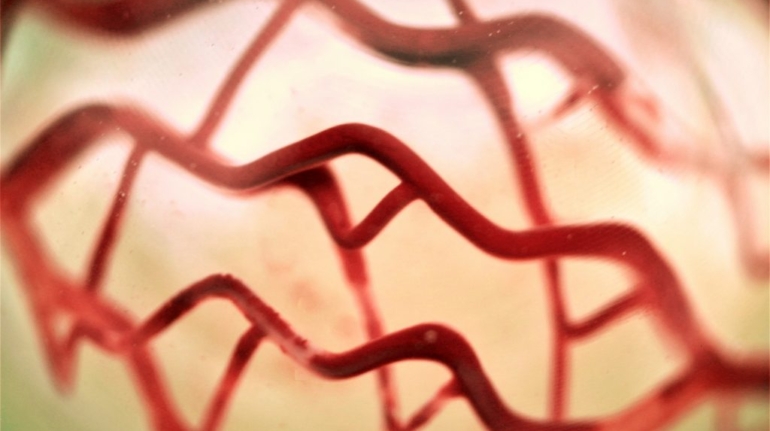
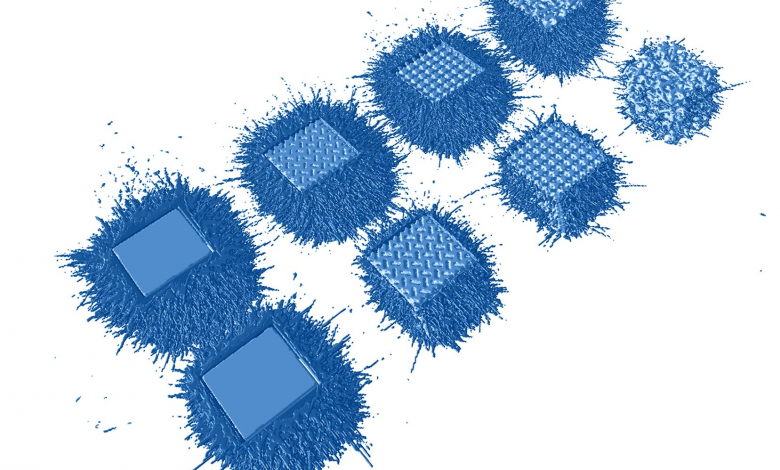
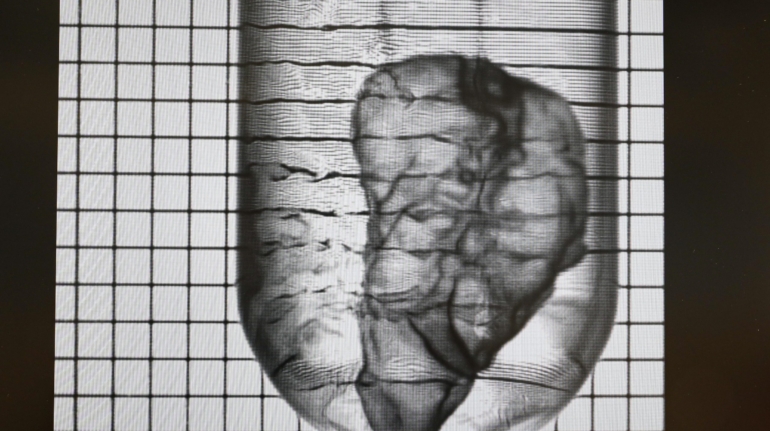
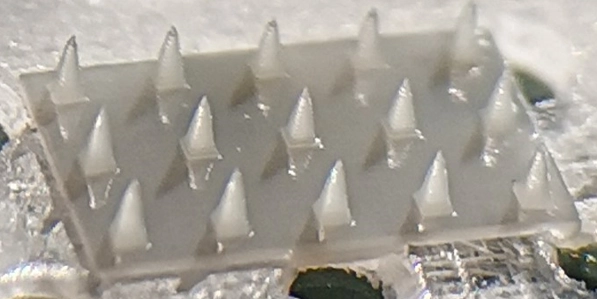
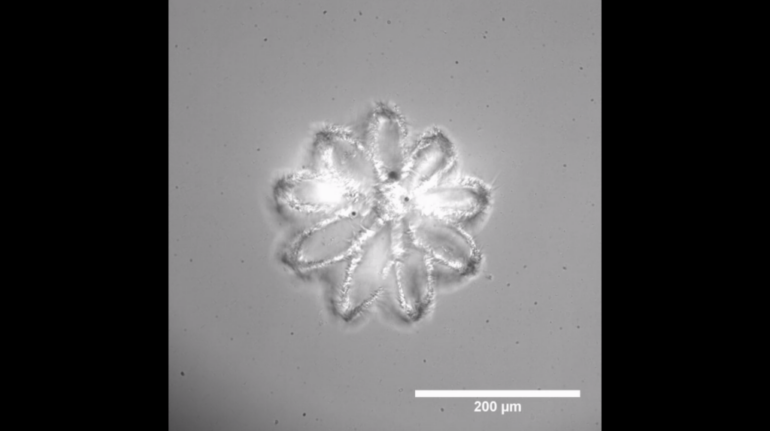
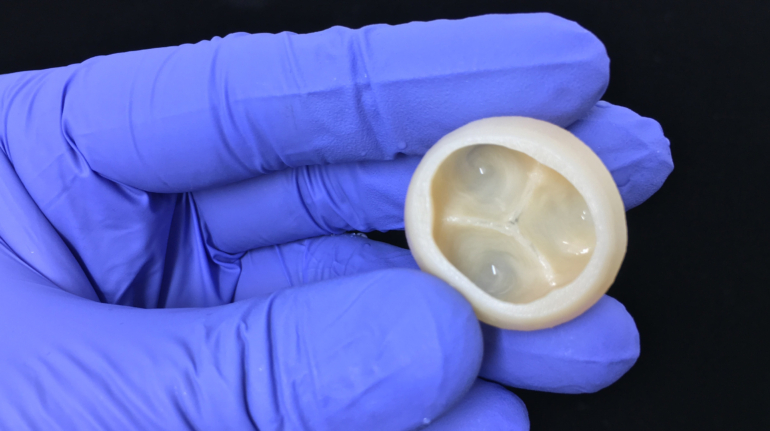
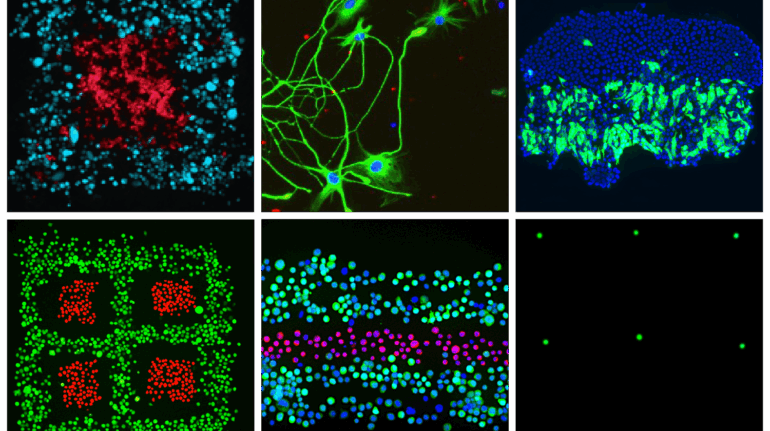
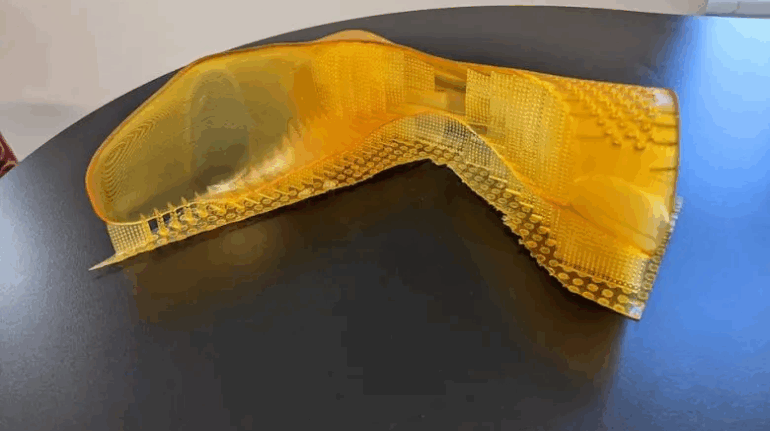
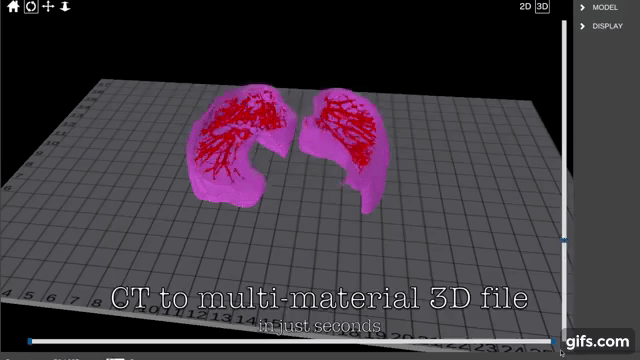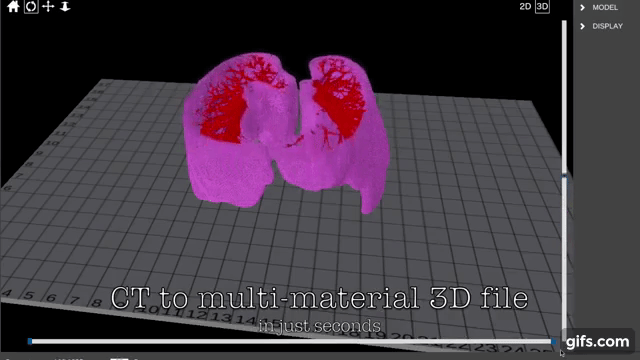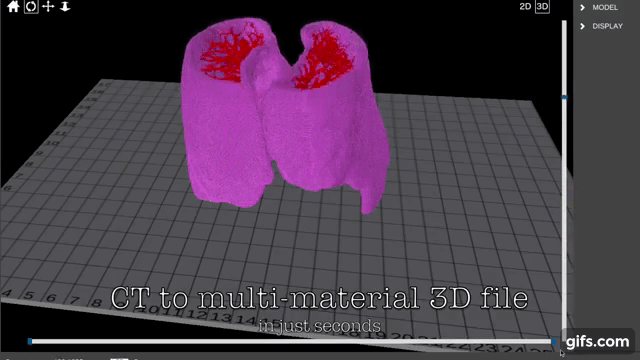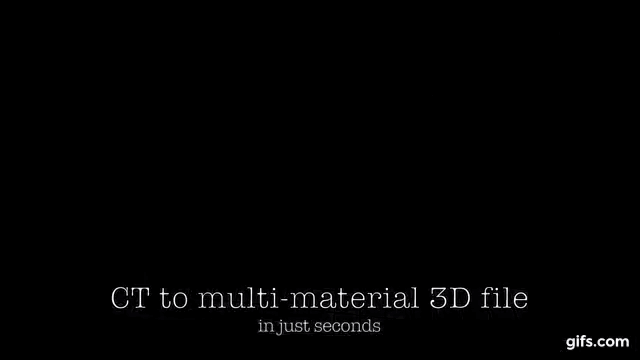
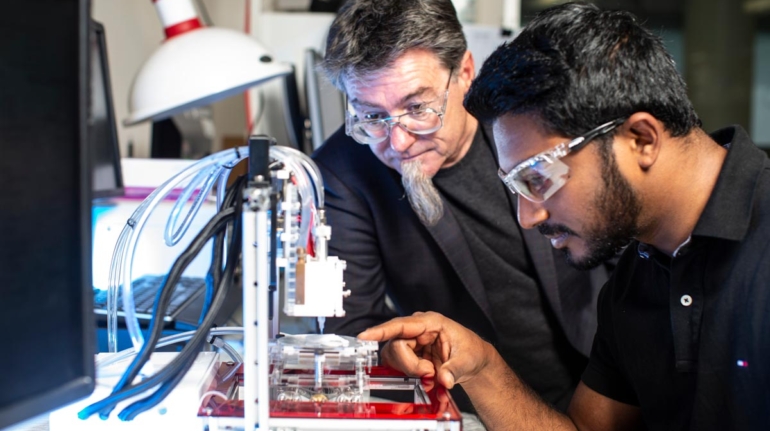
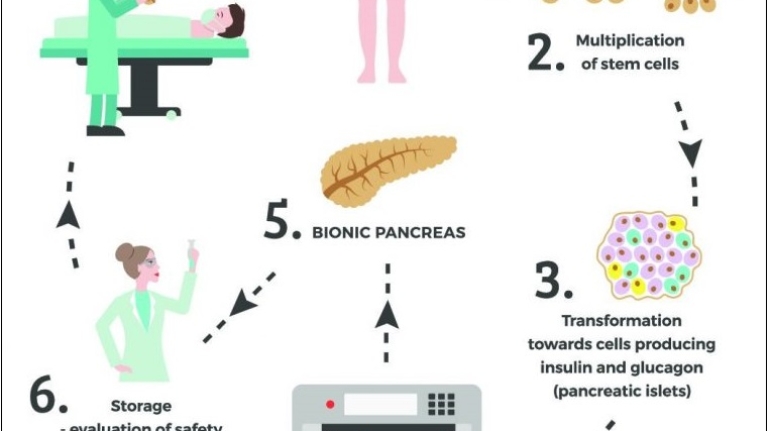
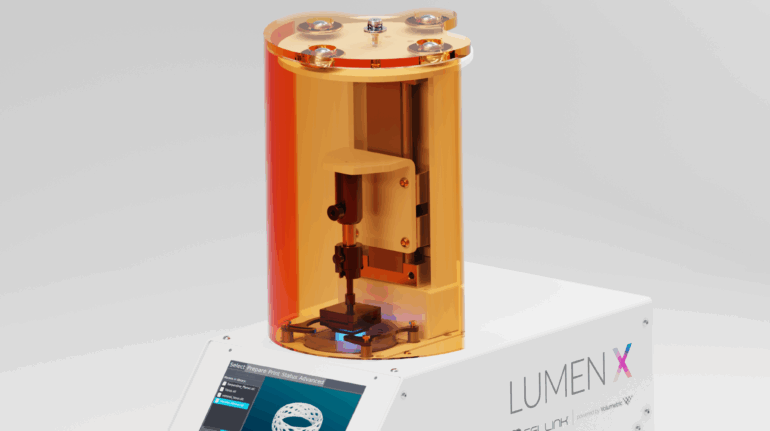
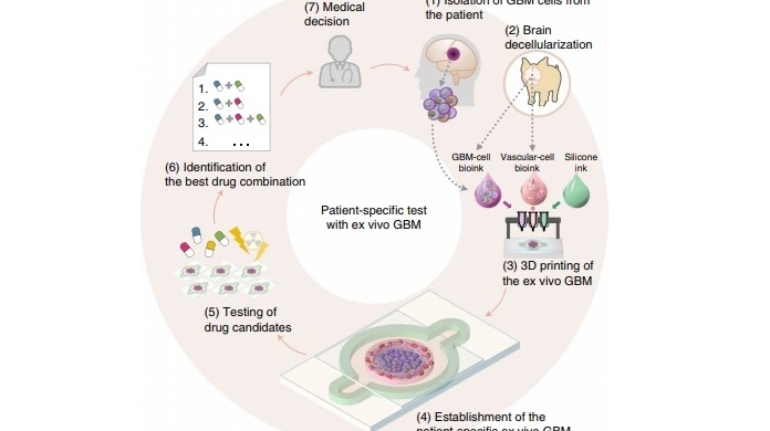
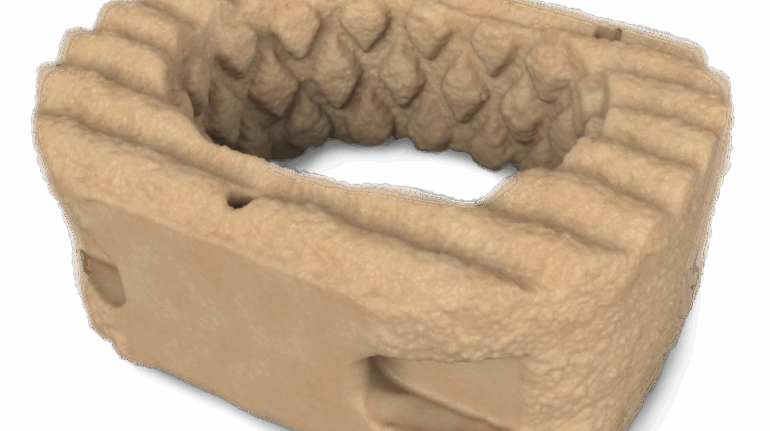
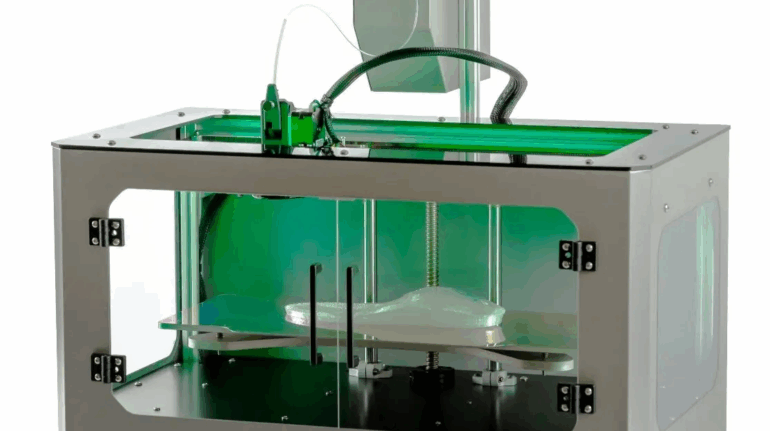
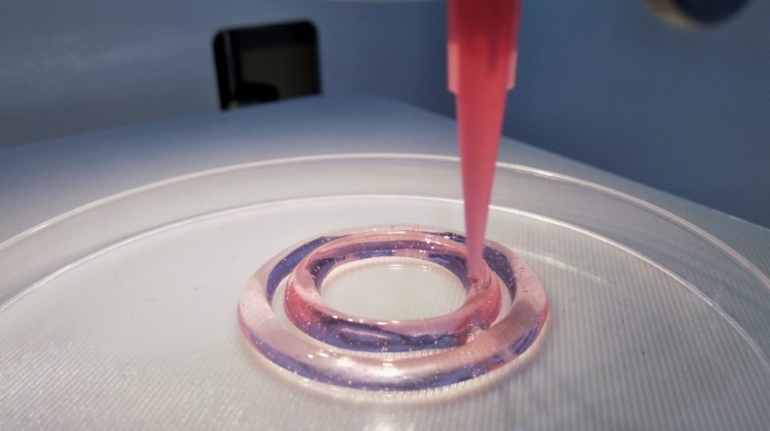
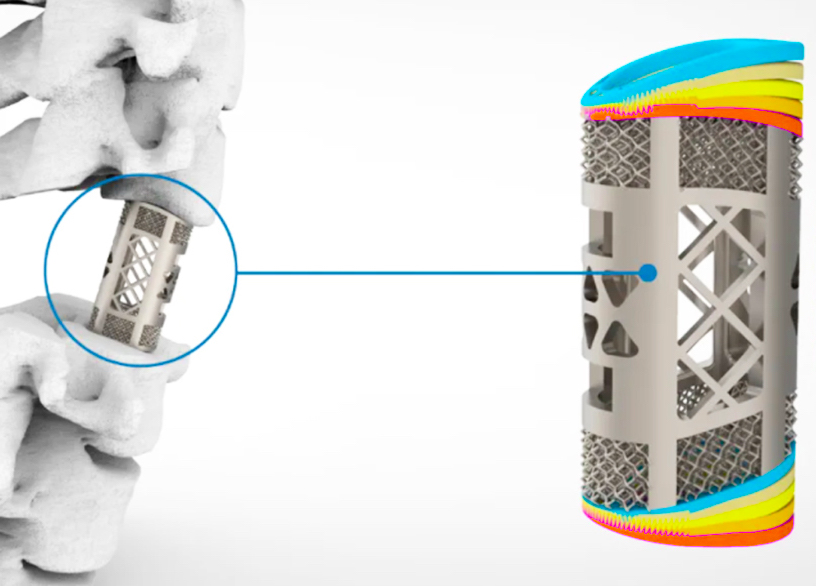
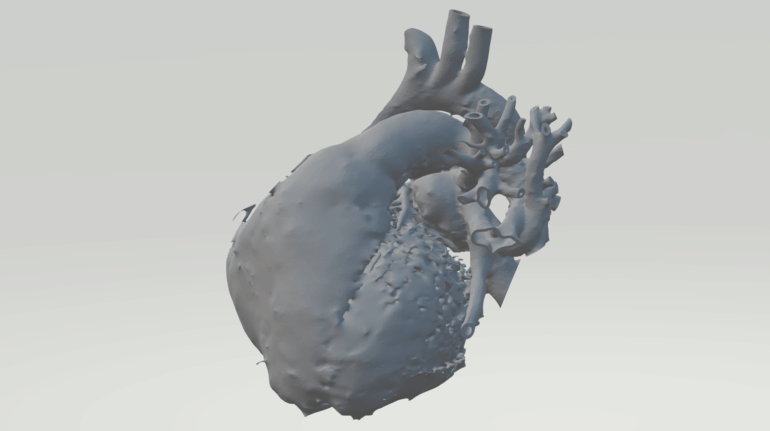
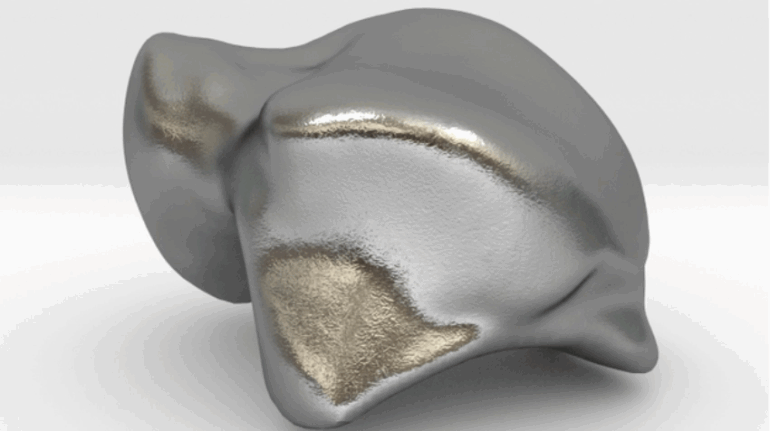
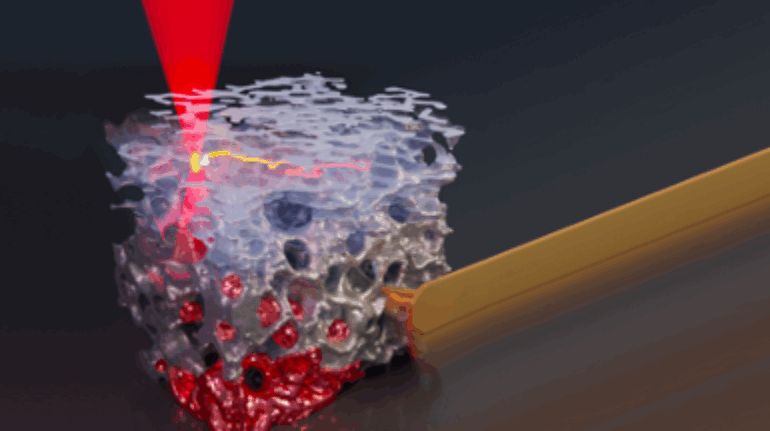
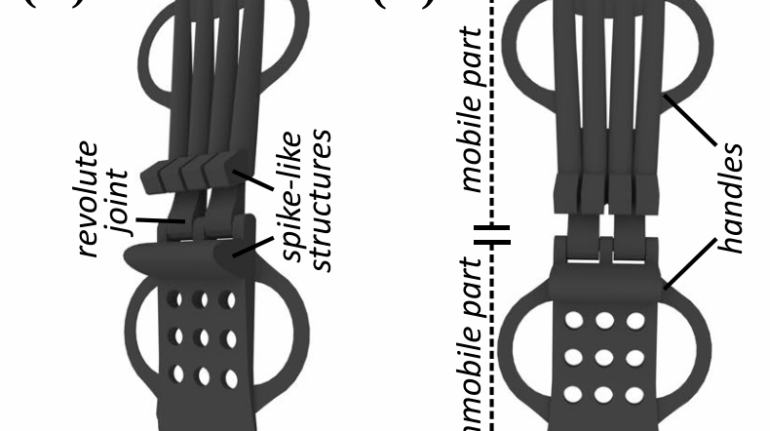
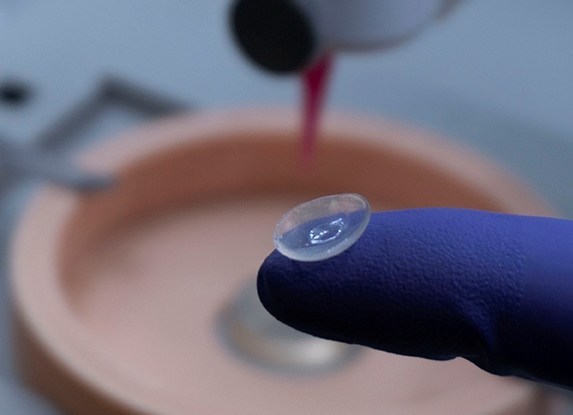
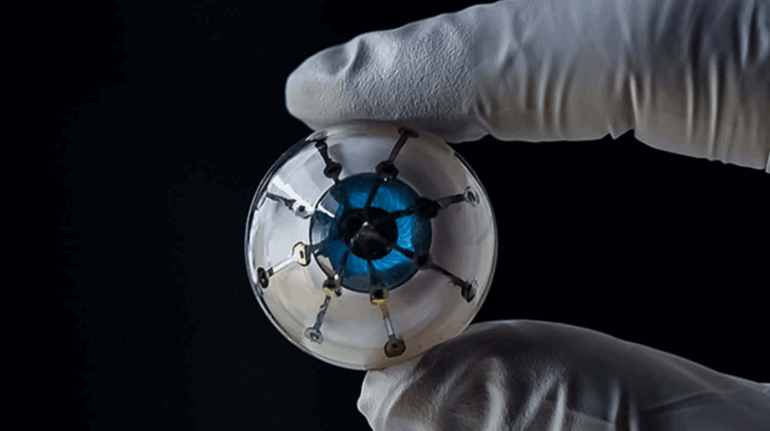
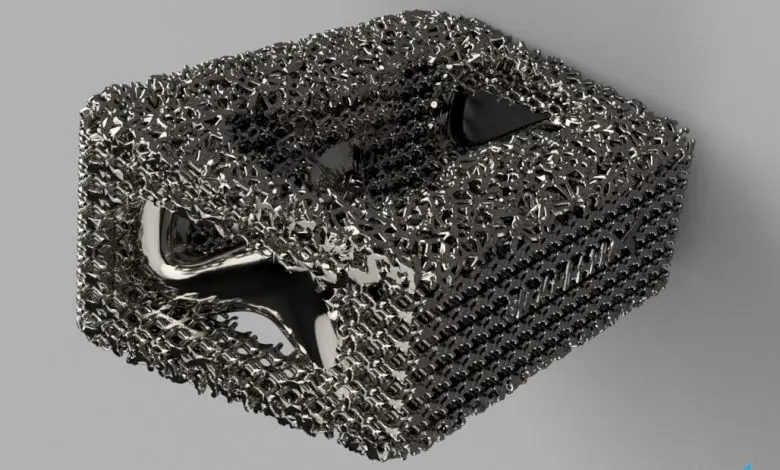
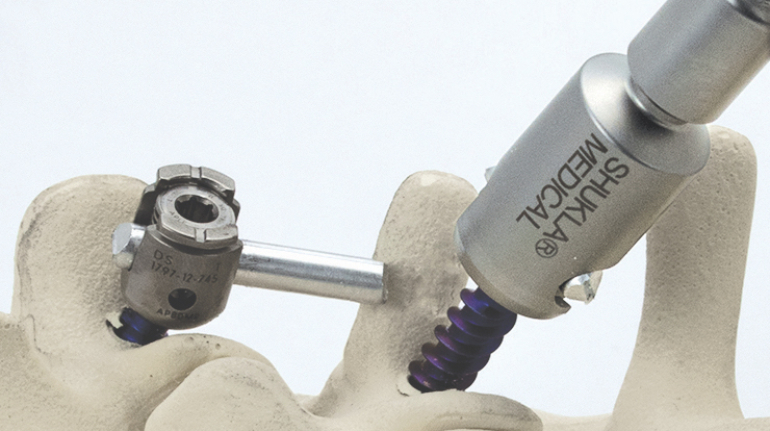
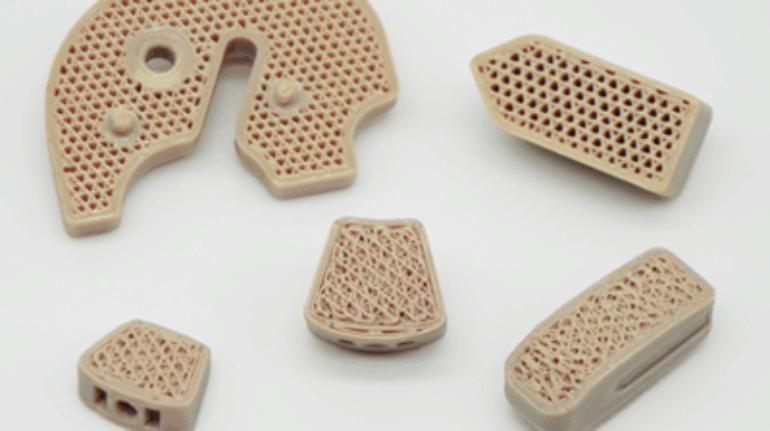
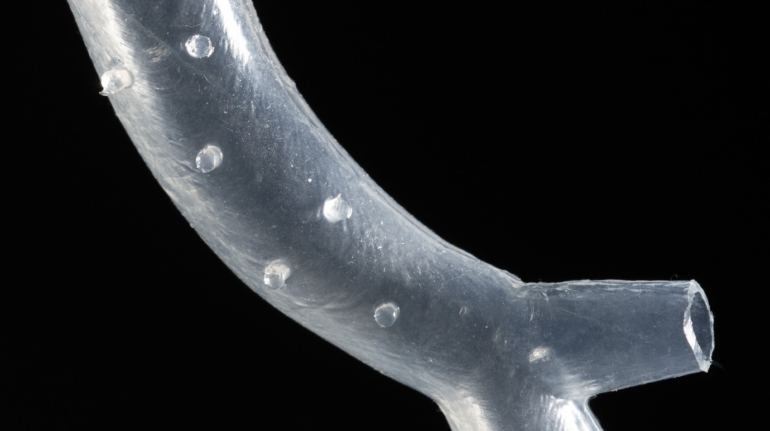
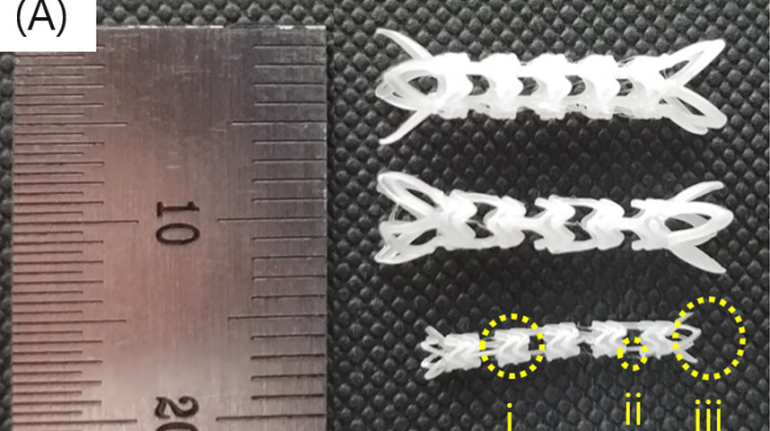
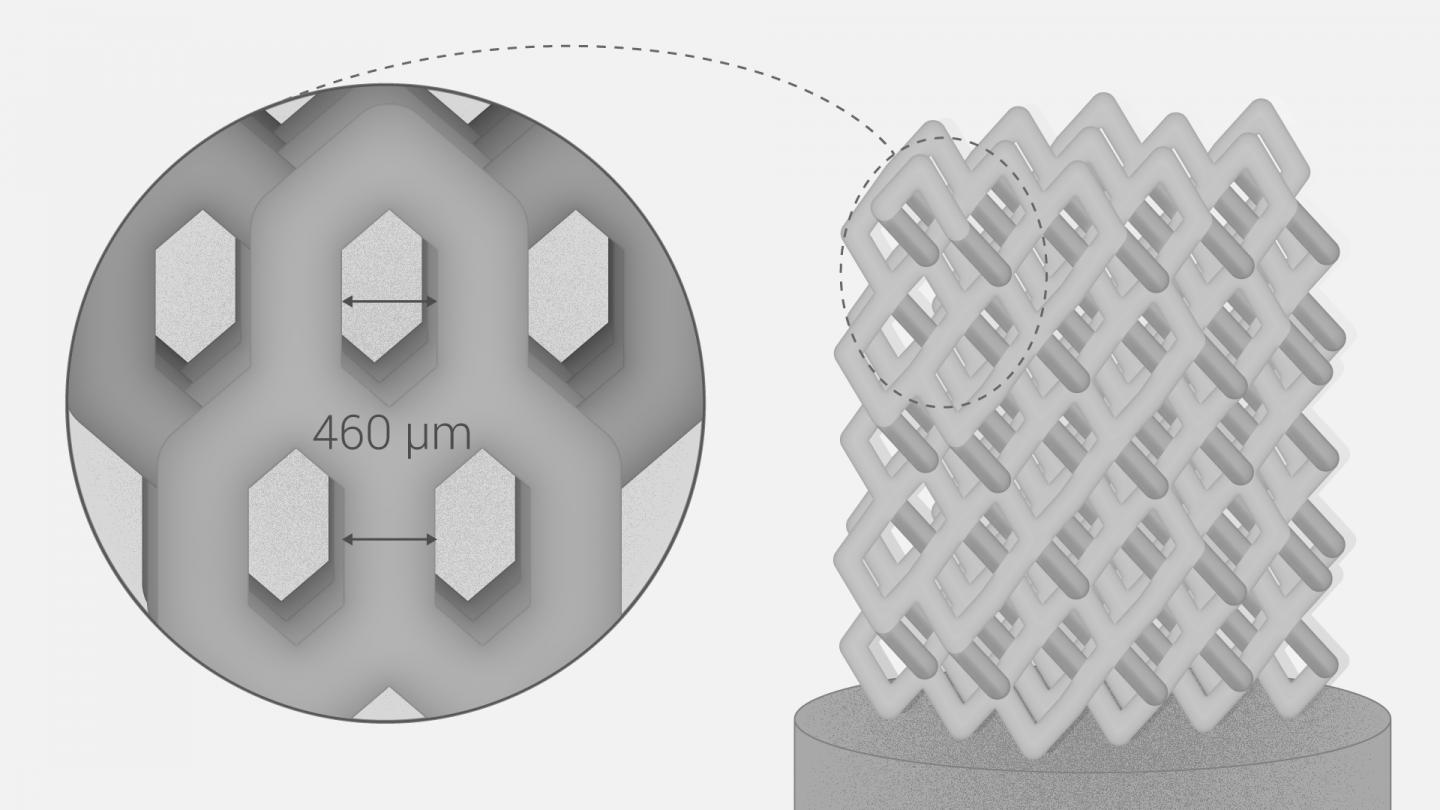
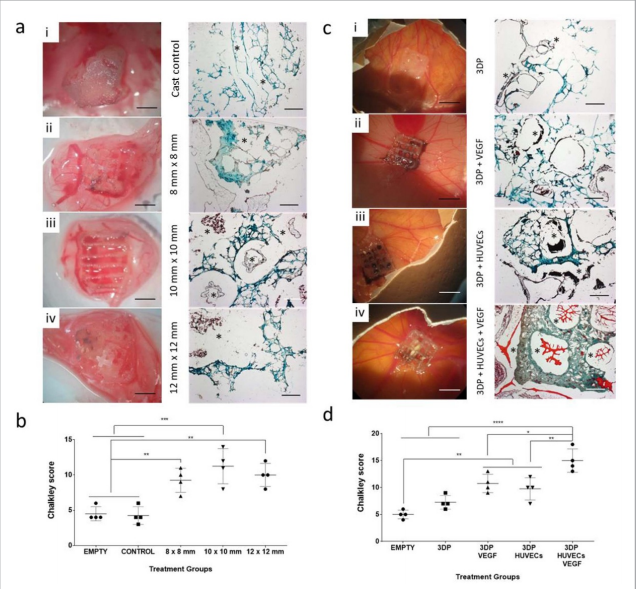
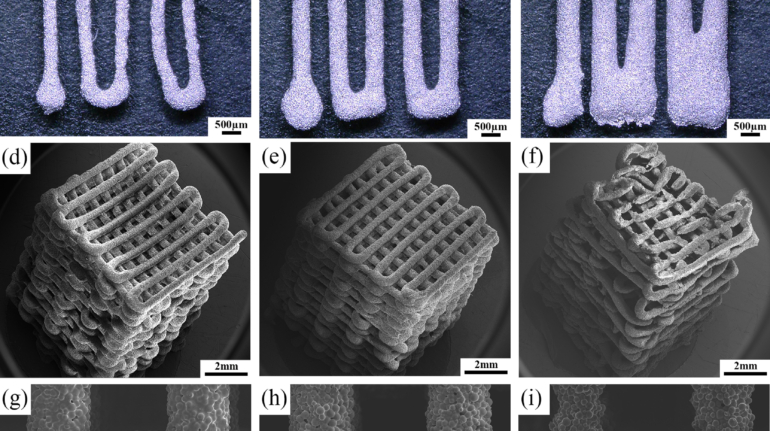
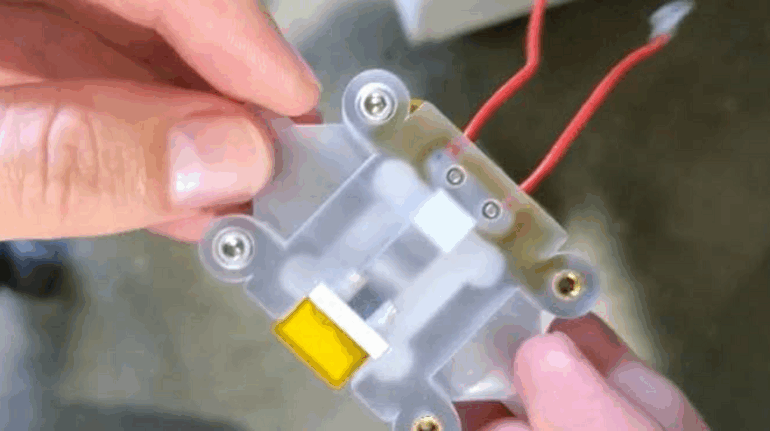
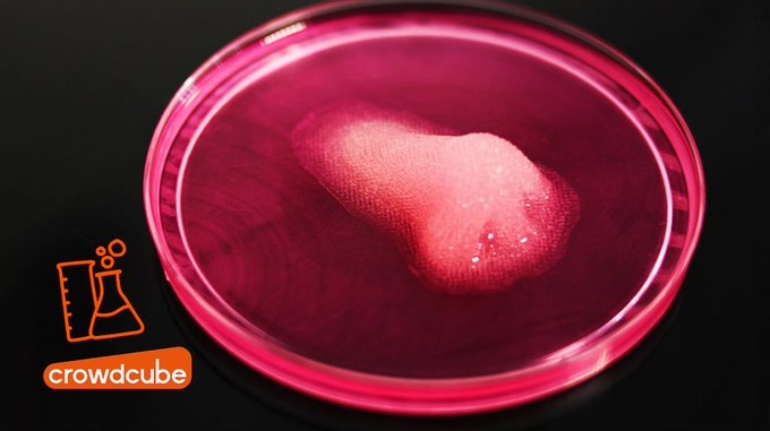
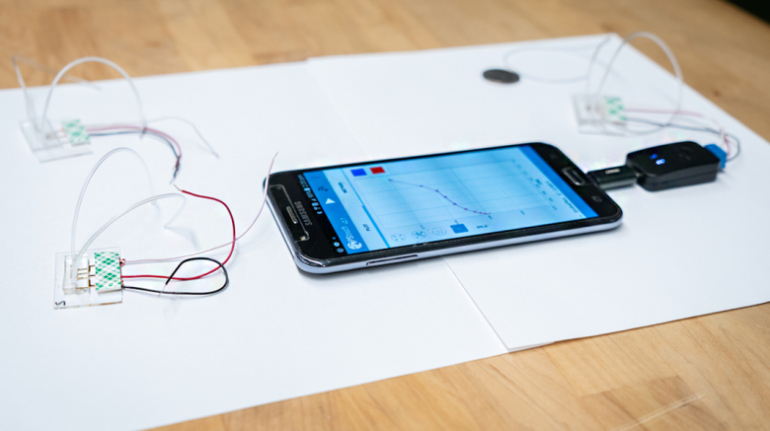
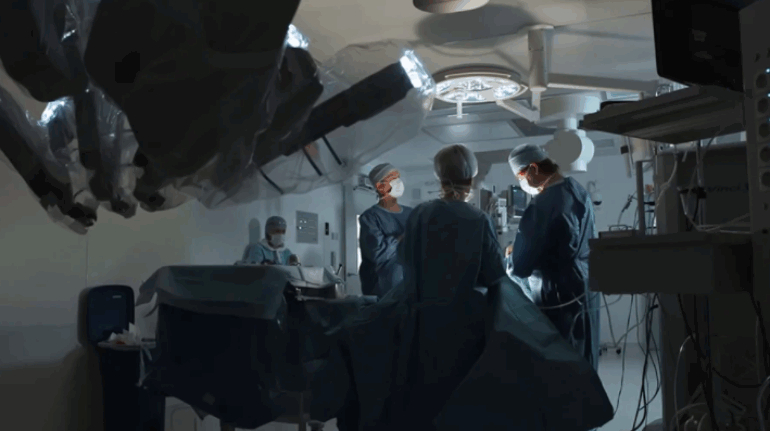
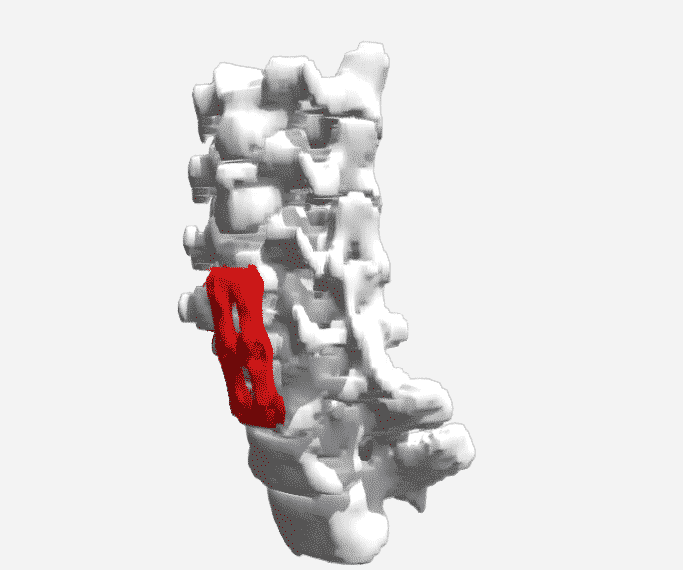
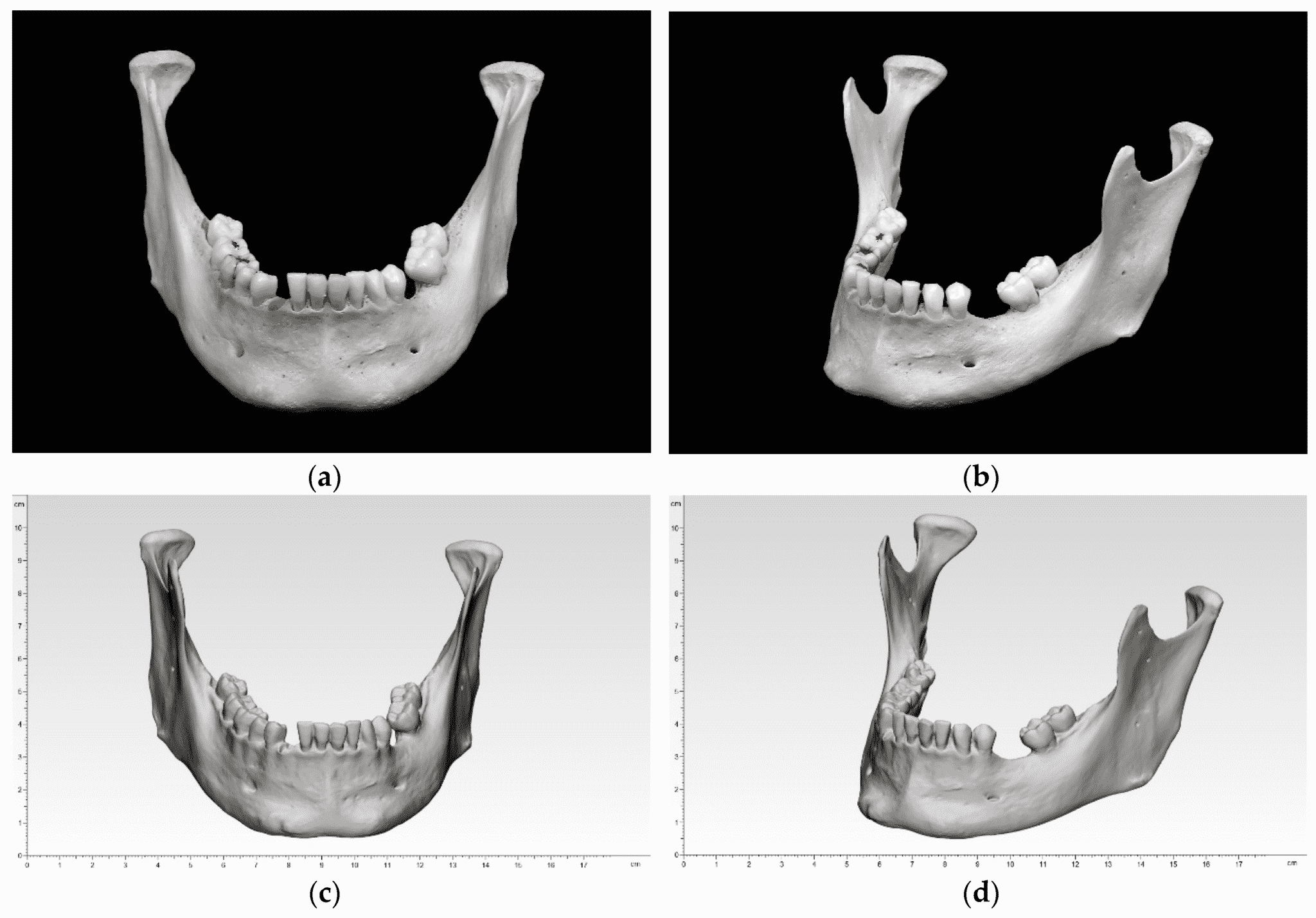
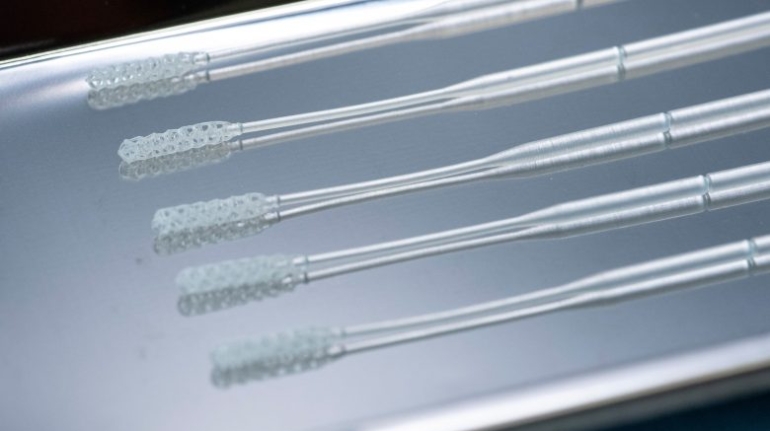
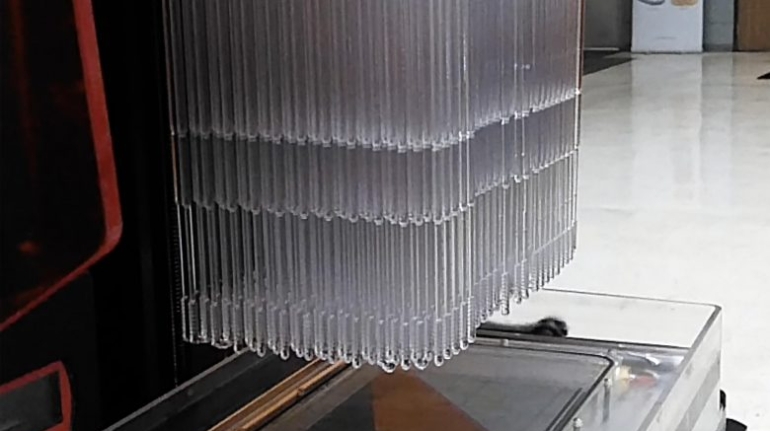
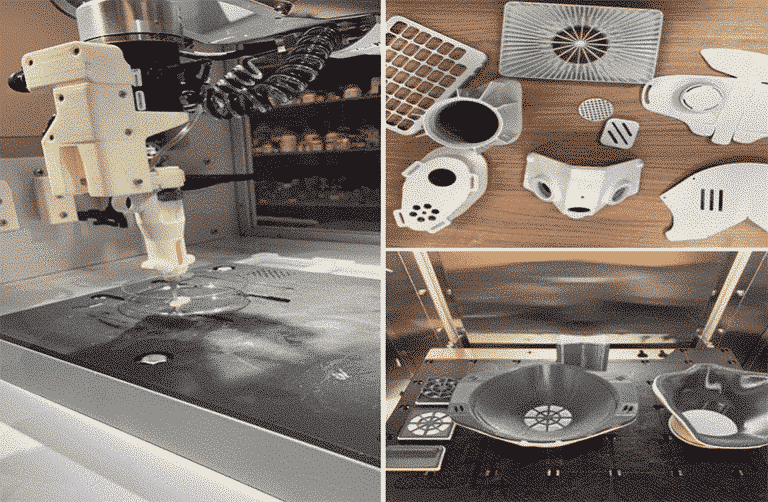
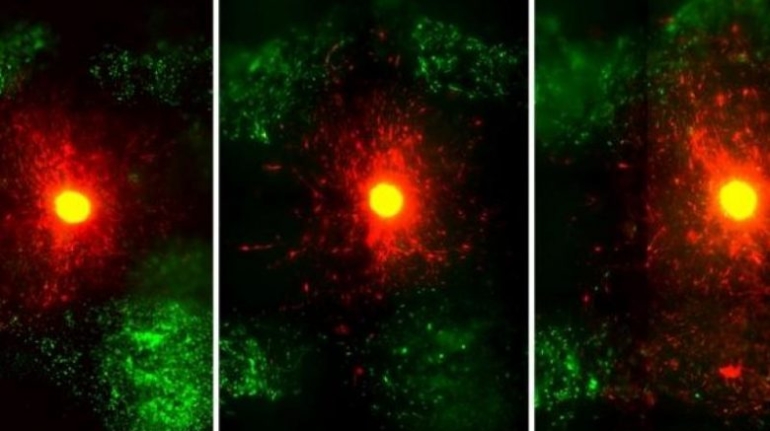
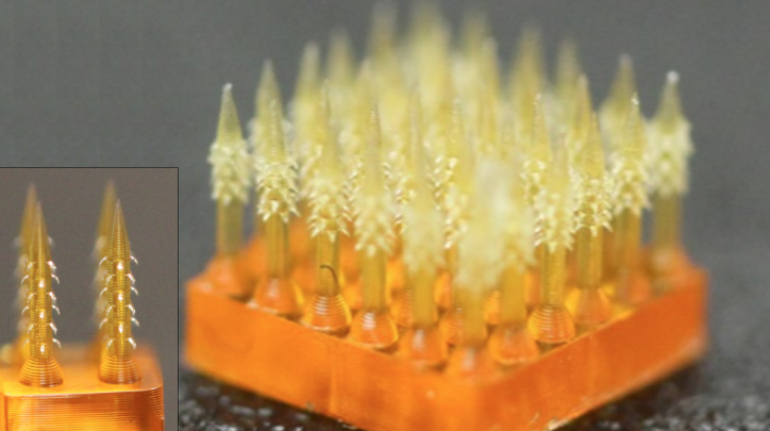
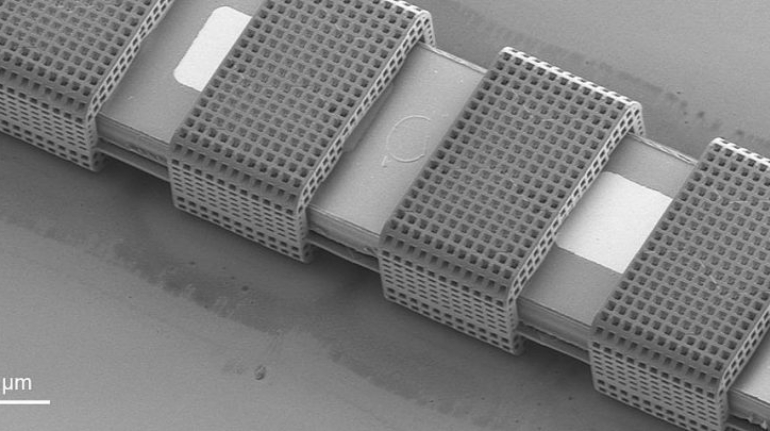
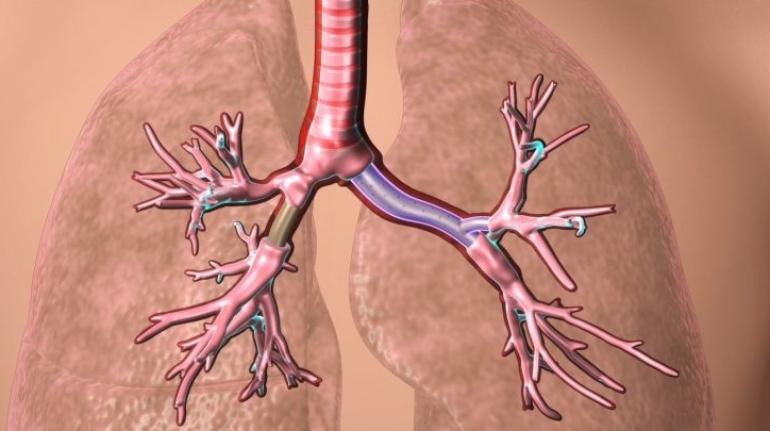
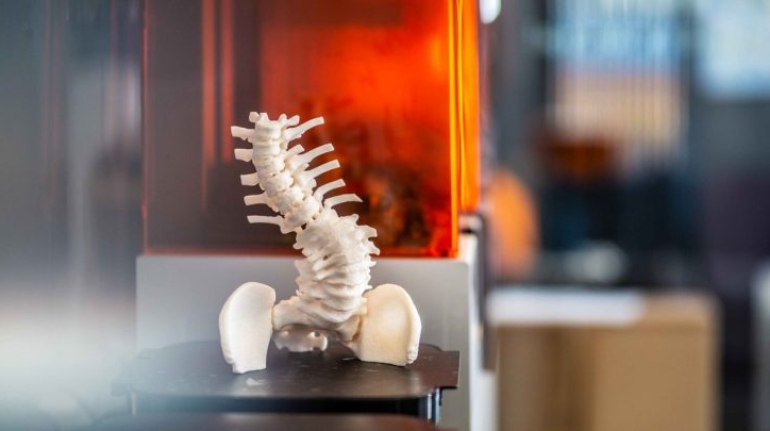
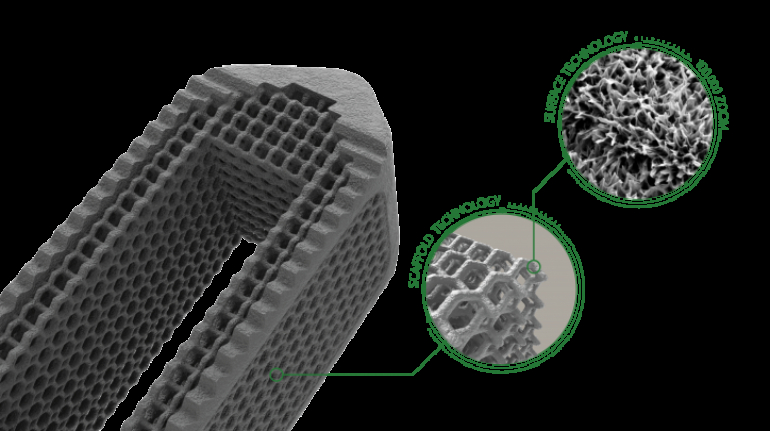
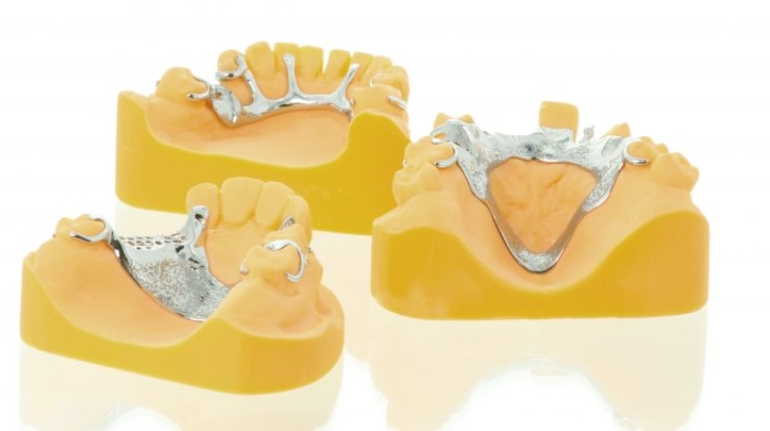
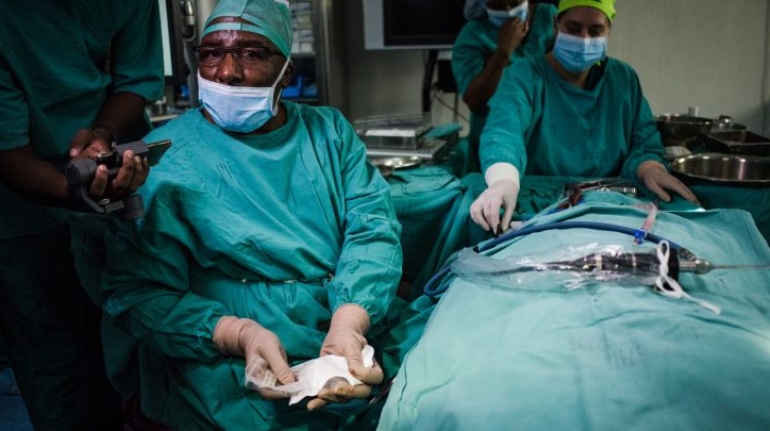
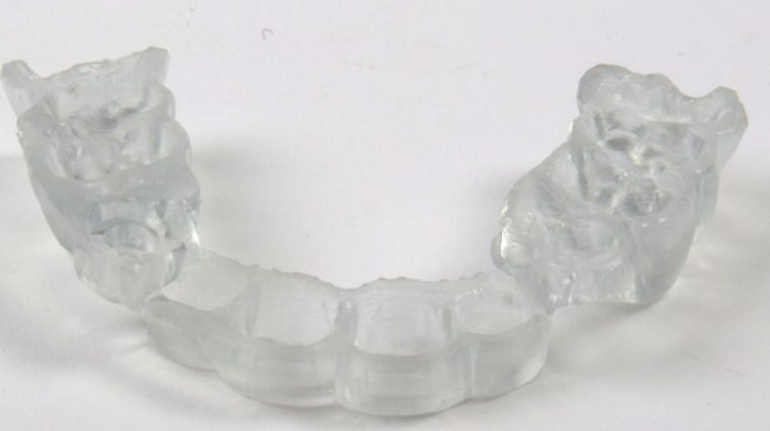
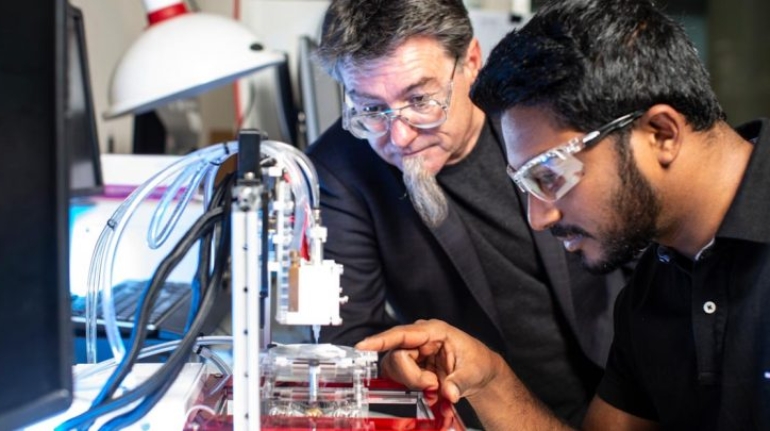
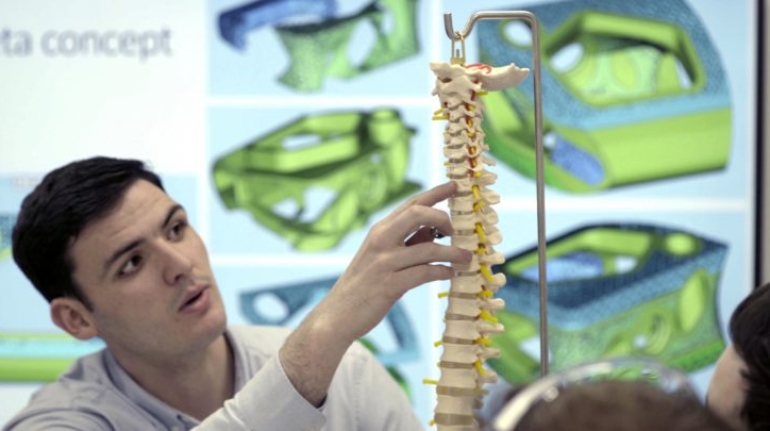
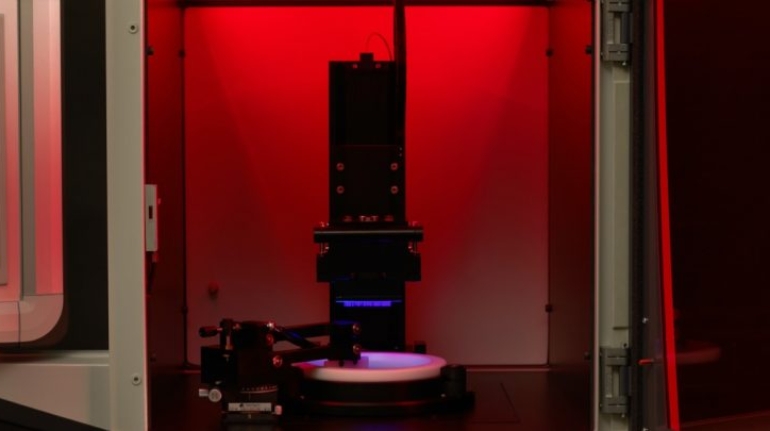
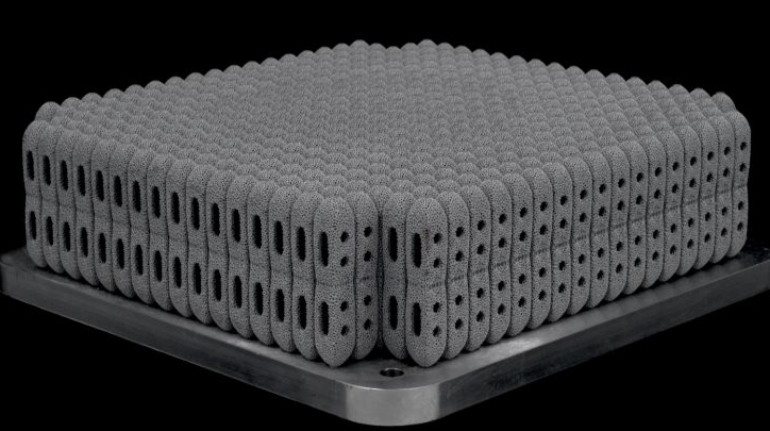
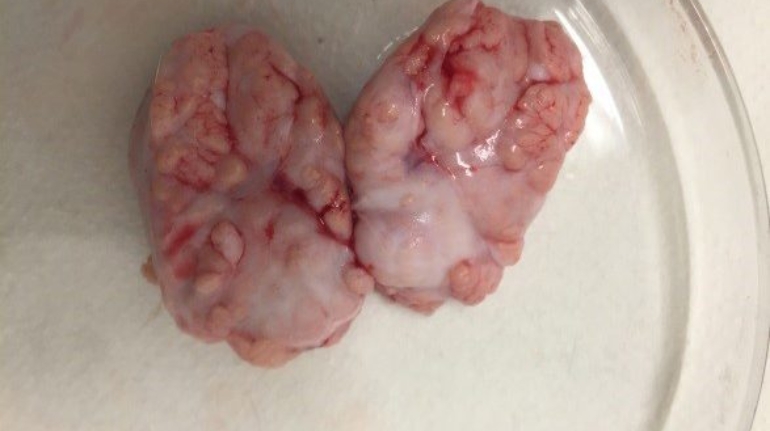
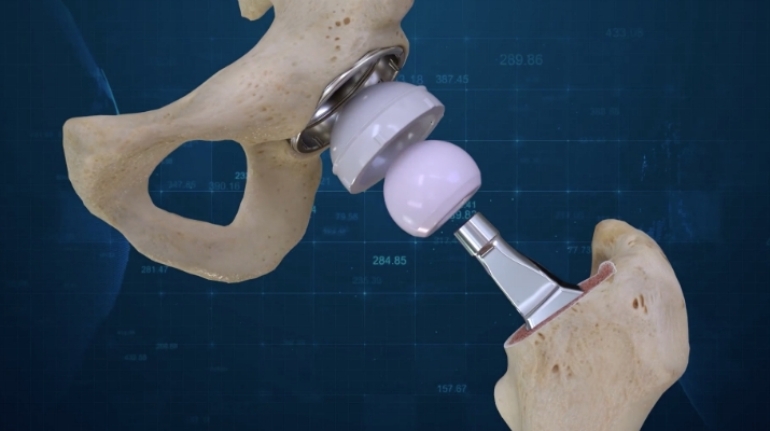
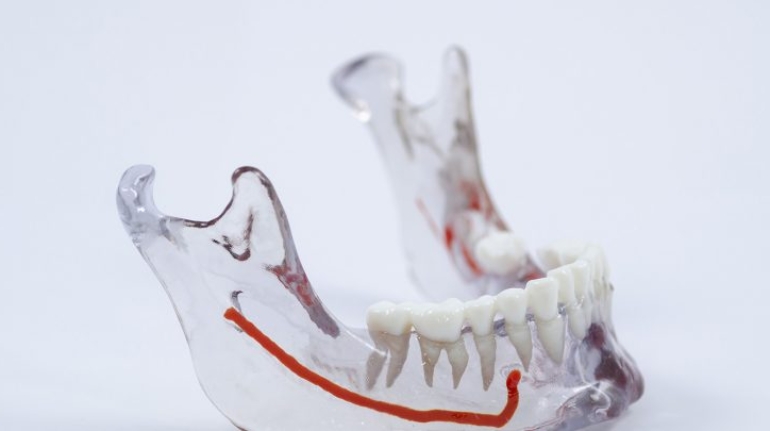
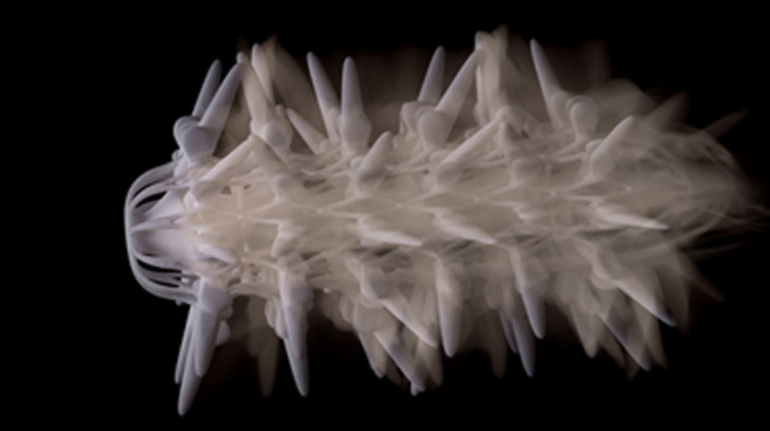
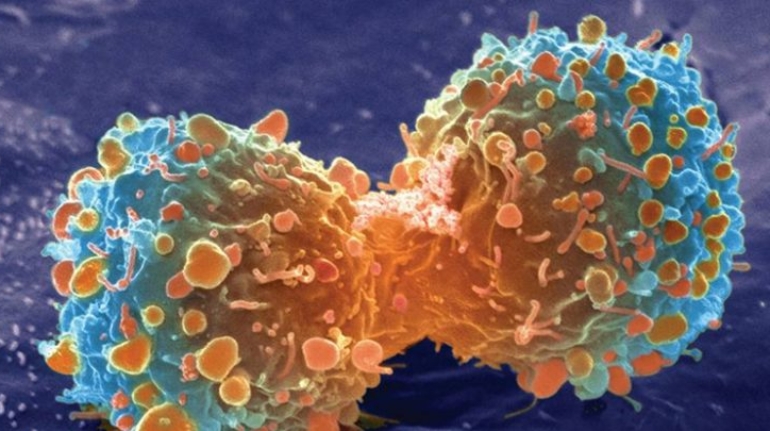
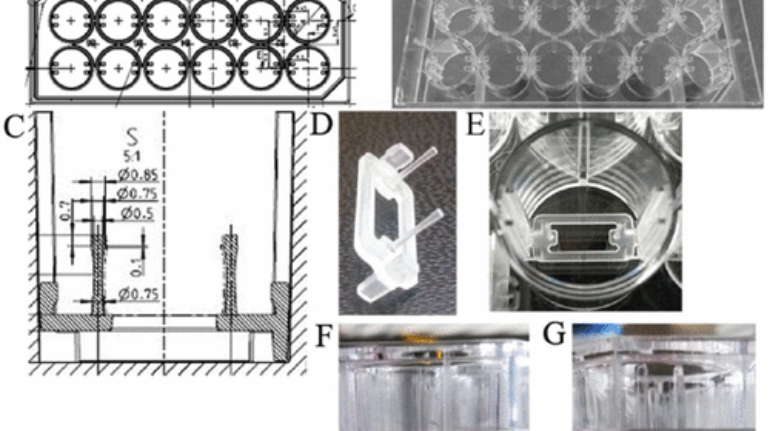
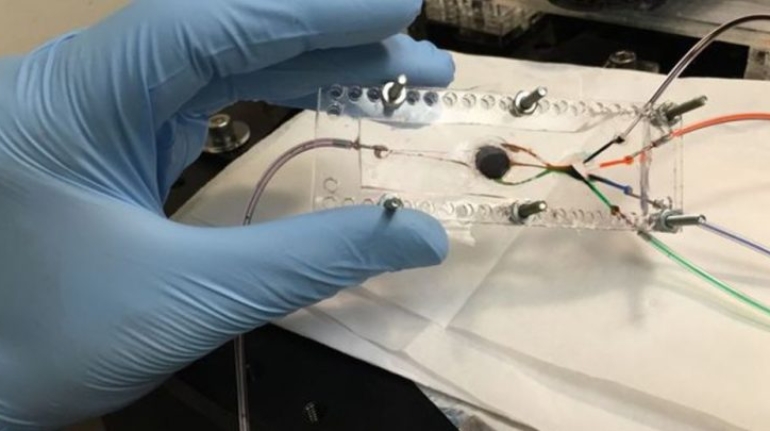
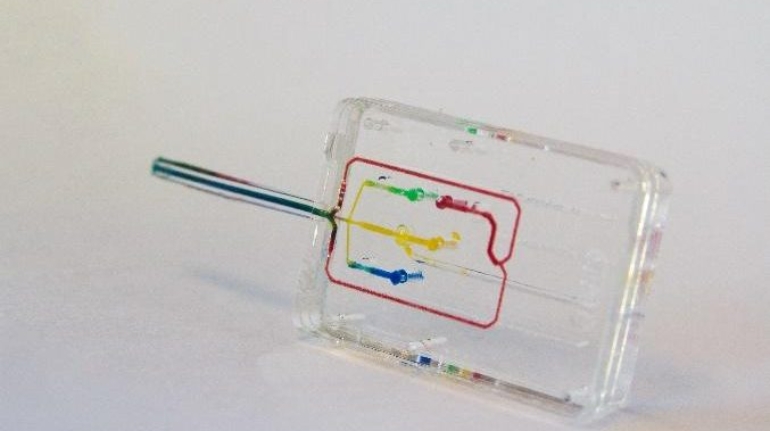
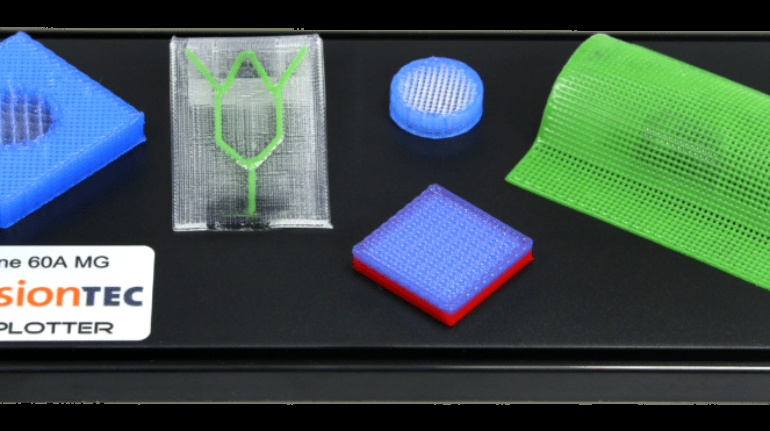
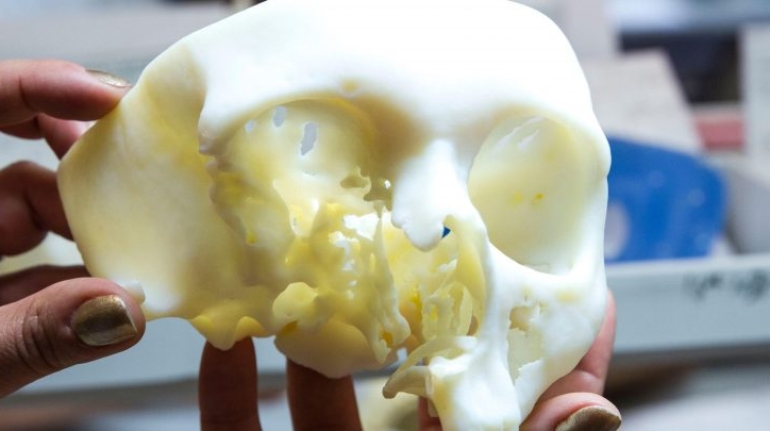
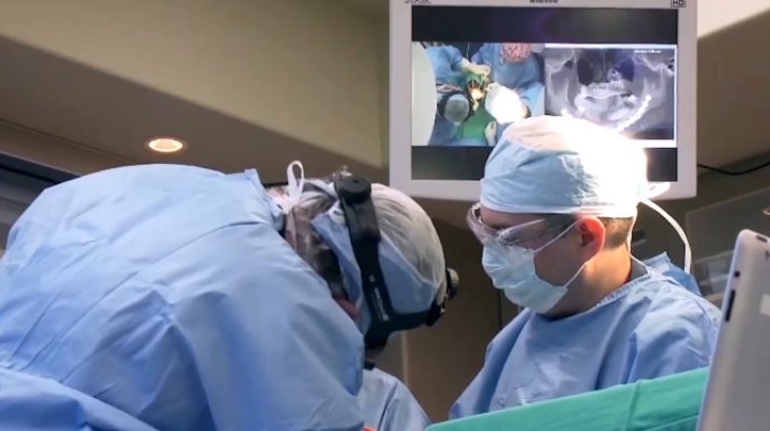
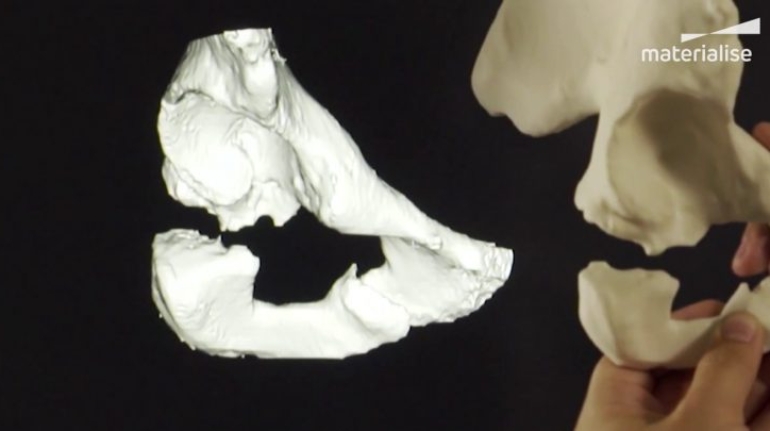
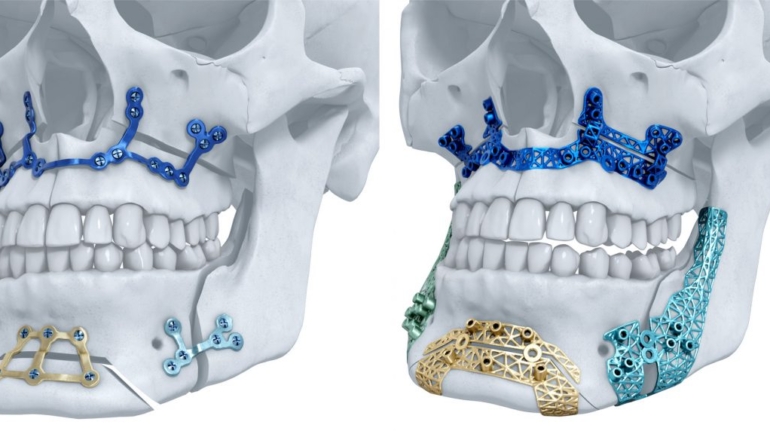
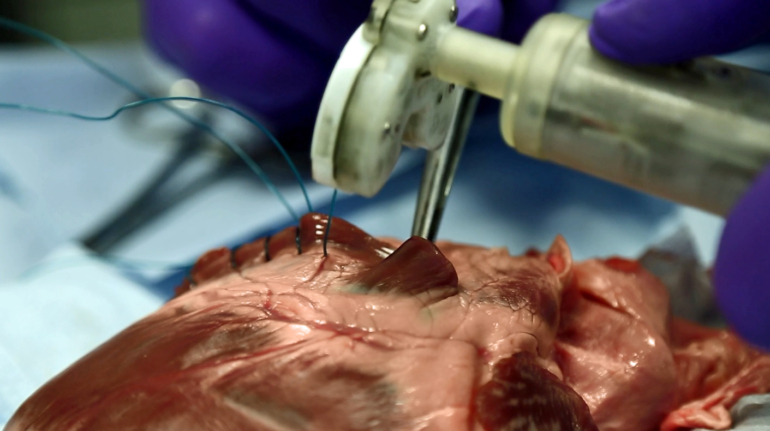
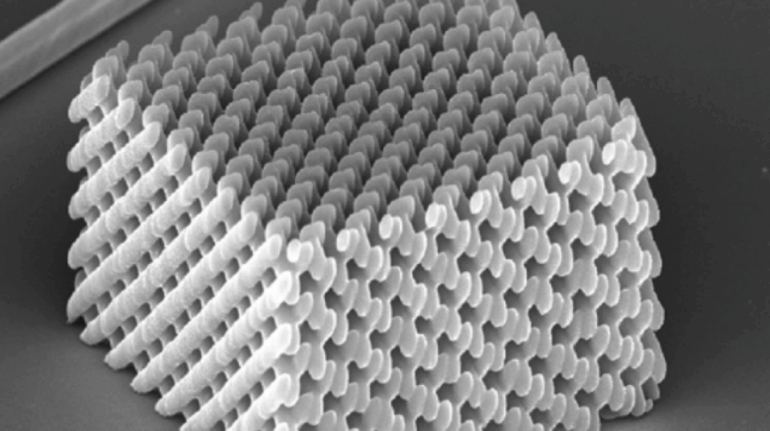
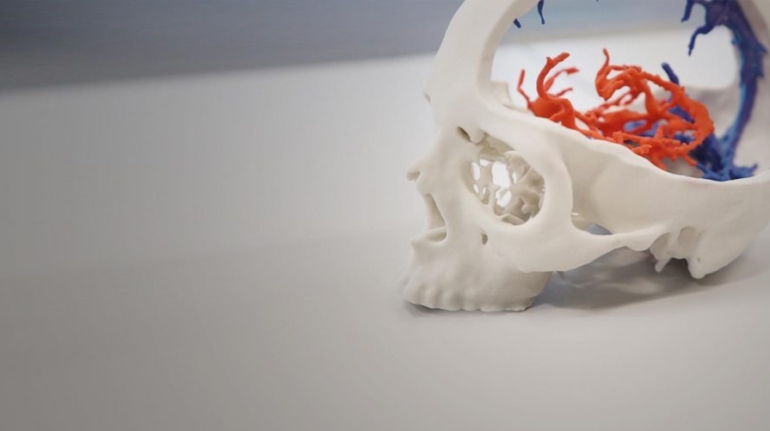
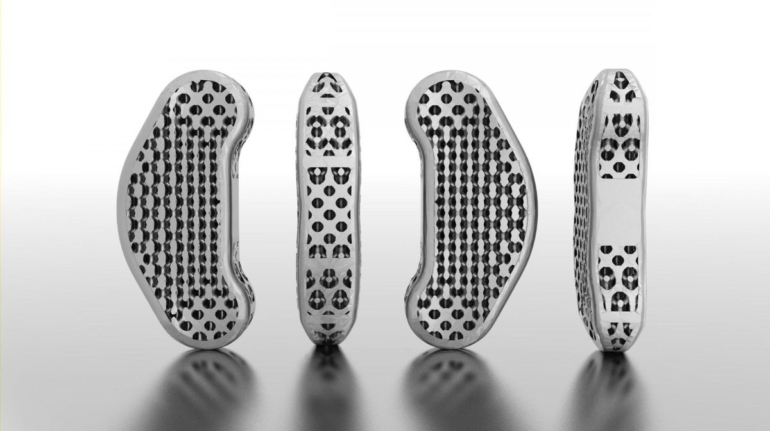
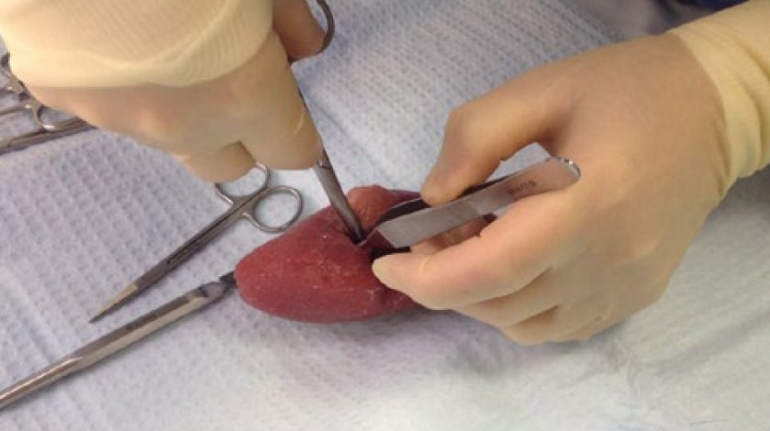
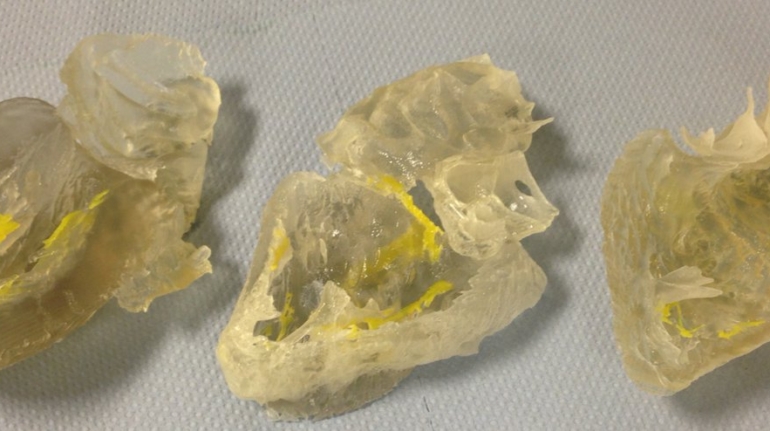
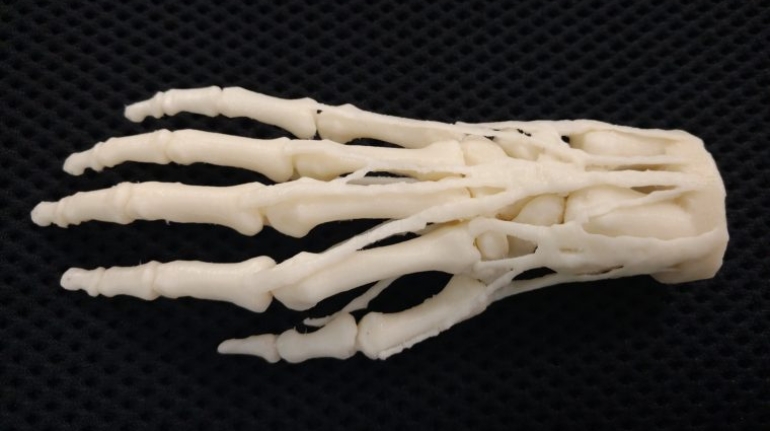
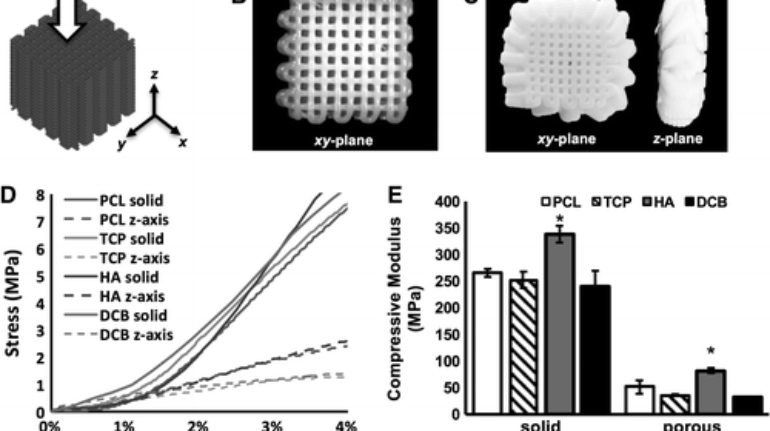
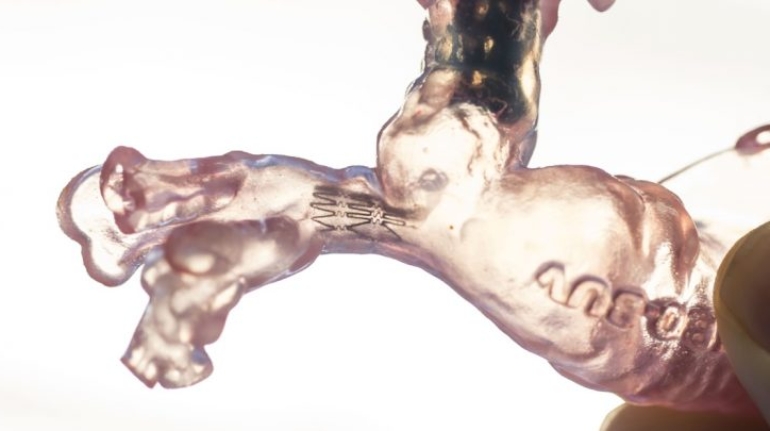
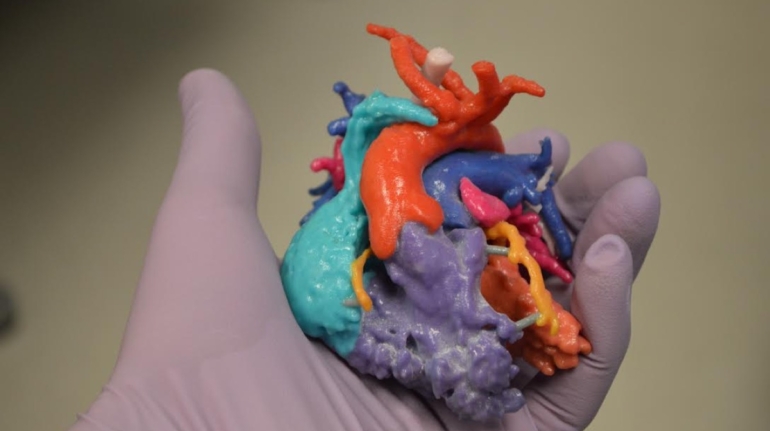
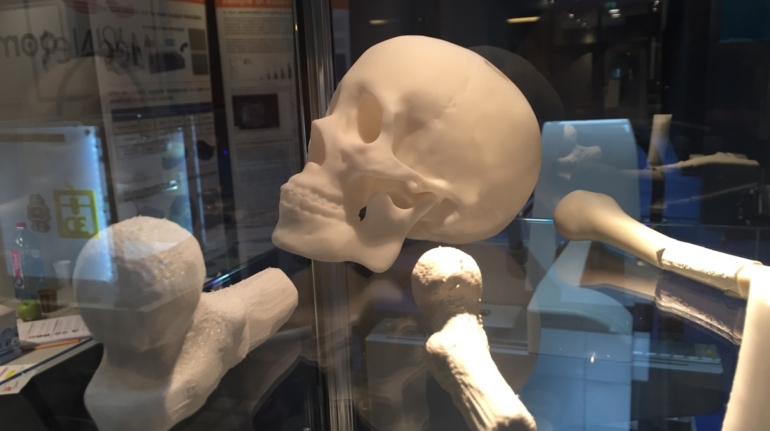
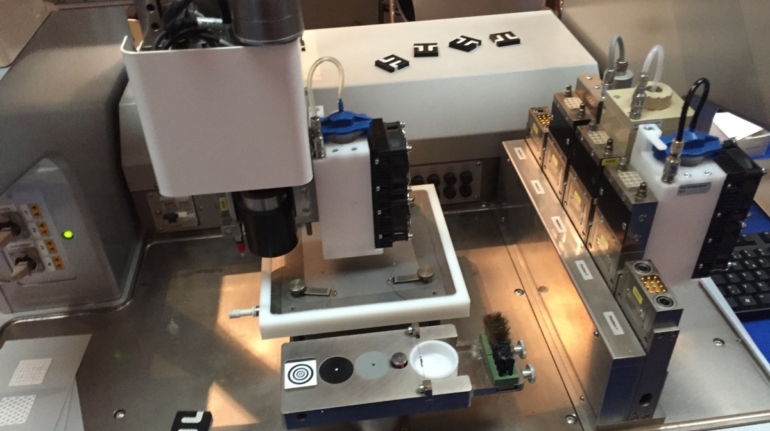
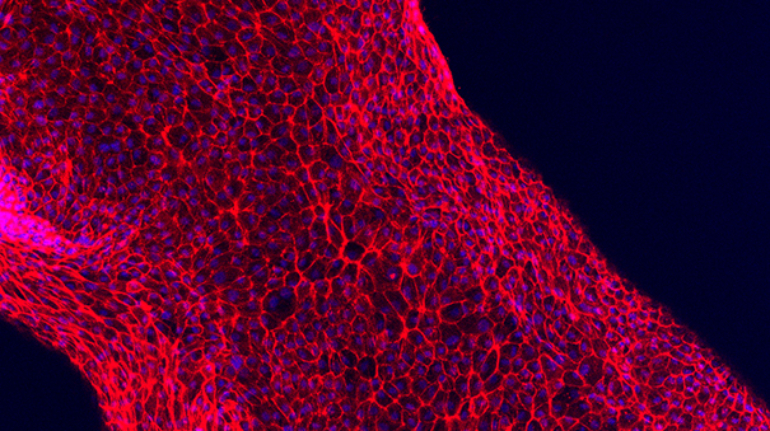
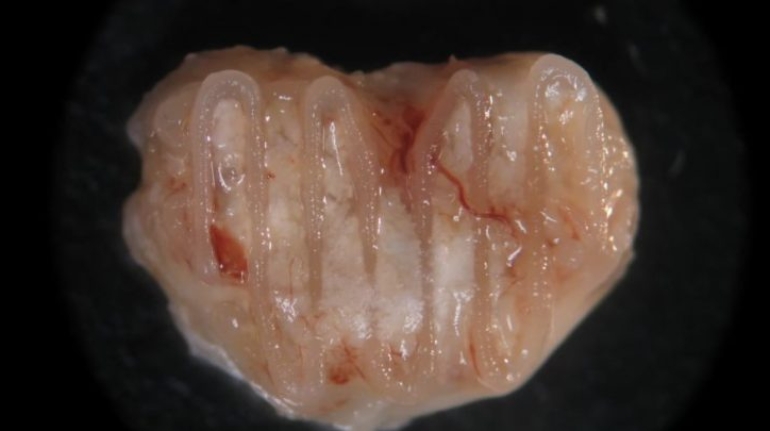
Researchers in AMBER, the Science Foundation Ireland funded materials science centre, hosted in Trinity College Dublin, have created a process to support 3D printing of new bone graft material. This world first research, led by Professor Daniel Kelly and recently published in the journal Advanced Healthcare Materials, could be used to regenerate large defects caused by tumour resections, trauma and infection, as well as inherited bone deformities. Professor Kelly’s research could also have numerous applications in craniomaxillofacial (the whole area of the mouth, jaw, face and skull) and orthopaedic surgery, especially in cases where tissues with complex geometries need to be regenerated, for example cases in the head, jaw or spine. Worldwide, 2.2 million procedures a year require a bone graft. At present there are currently two methods to provide a bone graft. The first is an autograft, where bone is transplanted from one site to another site within the same person. This type of grafting can be quite painful, and issues can arise at the site of extraction, as it heals. The second, an allograft is where bone is taken from a donor and transplanted. Complications can include donor site morbidity, poor availability of transplantable tissue and disease transfer from the donor to the recipient. AMBER’s new 3D printing method could replace traditional methods and eliminate these difficulties, by enabling the printing of larger and more complex shaped implants. Furthermore, the mechanical properties may be tailored for specific applications, which means bone grafts could be used in more complex cases such as in the head and jaw. AMBER researchers’ method consists of using 3D bioprinting technology to fabricate cartilage templates which have been shown to assist the growth of a complete bone organ. The AMBER team used 3D bioprinting to deposit different biomaterials and adult stem cells in order to engineer cartilage templates matching the shape of a segment within the spine. The team implanted the templates under the skin, where they matured over time into a fully functional bone organ with its own blood vessels. During skeletal development many of our bones are formed by a process in which cartilage templates are transformed into a vascularised and functioning bone organ. Professor Daniel Kelly, Investigator at AMBER and Director of the Trinity College Centre for Bioengineering, said: “This is new approach to tissue and organ engineering and we’re very excited. 3D bioprinting is a rapidly expanding area in the fields of tissue engineering and regenerative medicine. While the technology has already been used to engineer relatively simple tissues such as skin, blood vessels and cartilage, engineering more complex and vascularised solid organs, such as bone, is well beyond the capabilities of currently available bioprinting technologies. Our research offers real hope in the future for patients with complex bone trauma or large defects following removal of a tumour. In addition, this bioprinting approach could also be used in the development of the next generation of biological implants for knee and hip replacements. Our next stage of this process is to aim to treat large bone defects and then integrate the technology into a novel strategy to bioprint new knees.” Professor Kelly will be presenting his research at the 5-Year Trinity Biomedical Sciences Institute (TBSI) Symposium on Monday September 5, where leading bioengineers, cancer scientists, clinicians and immunologists will discuss their next-generation research projects. For a full agenda of speakers, see:https://www.tcd.ie/biosciences/assets/pdf/agenda_tbsi_5anniversary_draft_tb.pdf A short video of the process can be found here, A short video of the process can be seen here,https://youtu.be/NWBa8OWgApM. The paper can be found in full here:https://onlinelibrary.wiley.com/doi/10.1002/adhm.201600182/full.