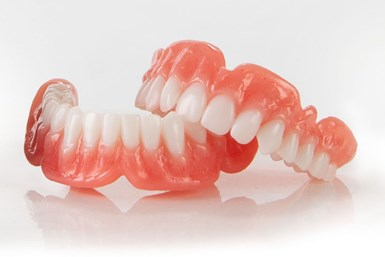
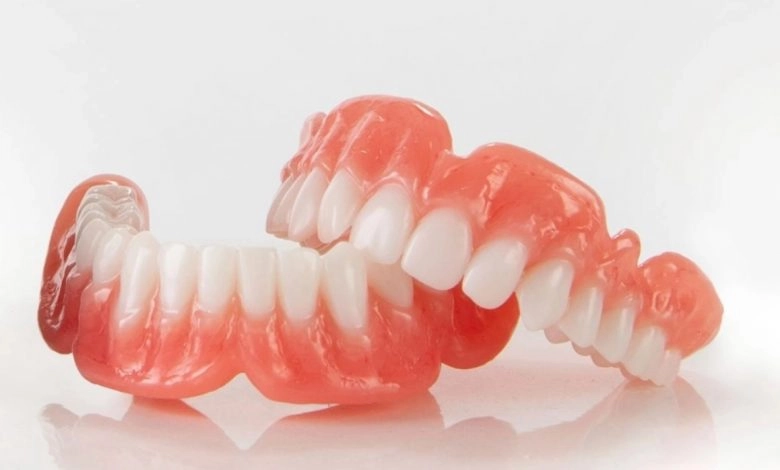
Desktop Health receives FDA clearance for Flexcera Medical
Desktop Health, recently launched by Desktop Metal, has received U.S. Food and Drug Administration (FDA) 510(k) clearance of FlexceraTM Base, a proprietary resin for use in 3D fabrication of high-quality dental prosthetics. Flexcera Base along with the new FlexceraTM Smile are Desktop Health’s first formulated and optimized digital dental solutions.