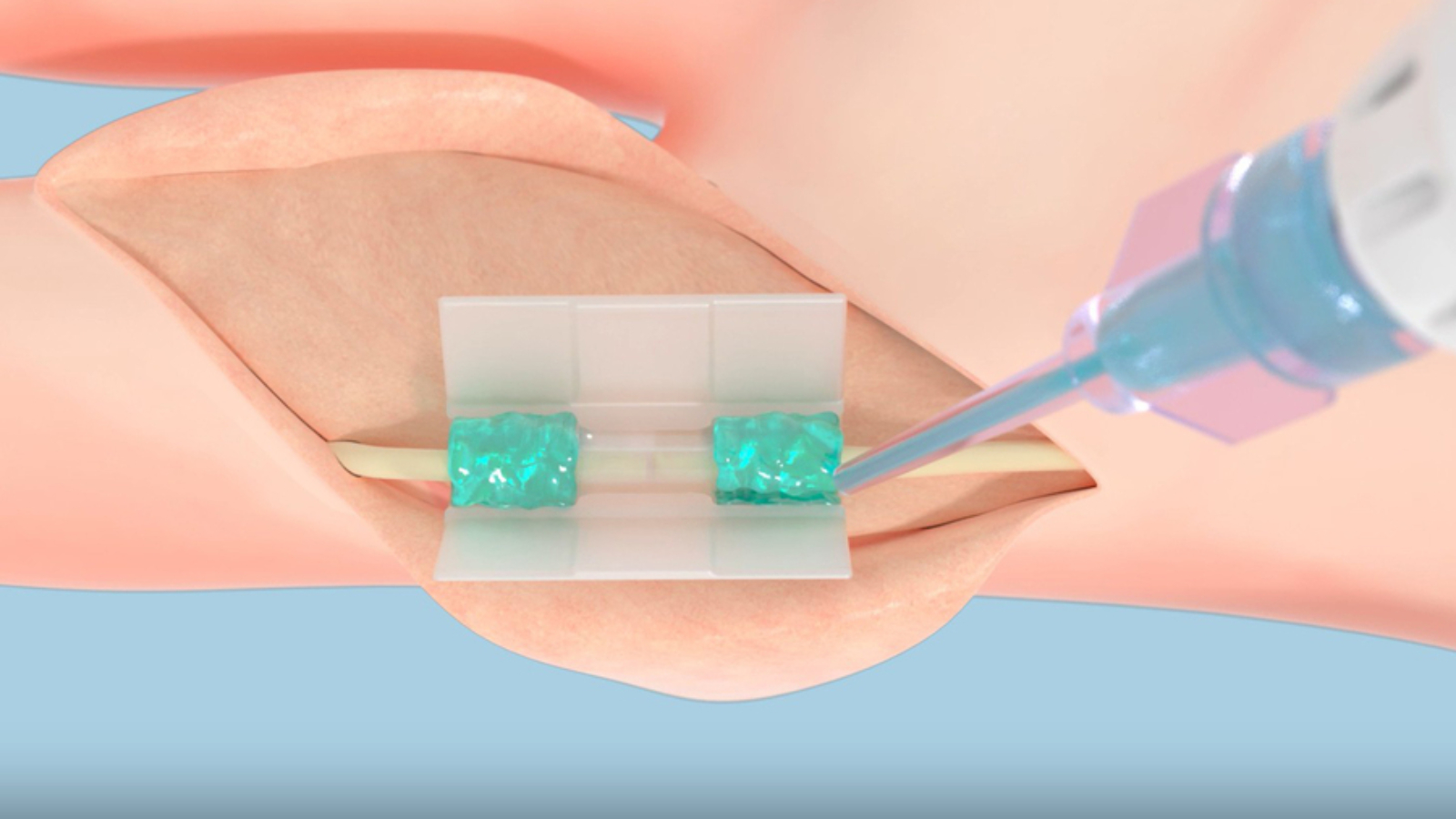
Tissium, a Paris-based medical technology company founded on MIT research, has secured De Novo marketing authorization from the U.S. Food and Drug Administration for its non-traumatic peripheral nerve repair platform. The clearance enables commercialization of its first product, which uses a flexible, biocompatible polymer that bonds to wet tissue when activated by blue light. In a recent clinical trial of 12 patients, all of whom completed follow-up, regained full flexion and extension of their injured digits, and reported no pain 12 months after surgery.
The authorization acknowledges the novelty of the company’s polymer platform, developed to avoid the trauma and variability associated with sutures, staples, and glues. “Our goal is to make this technology the new standard in fixation,” said co-founder Maria Pereira, who began working with polymers as a PhD student through the MIT Portugal Program. “Surgeons have been using sutures, staples, or tacks for decades or centuries, and they’re quite penetrating. We’re trying to help surgeons repair tissues in a less traumatic way.”
The biopolymers originated in the lab of MIT Institute Professor Robert Langer, where postdoctoral researcher Jeff Karp created biodegradable, photocurable elastic materials. Pereira optimized the platform’s thickness and water-repelling properties to adhere securely to wet tissue. Cardiac surgeon Pedro del Nido at Boston Children’s Hospital identified congenital heart defects as an urgent use case, and Pereira led preclinical work sealing holes in rat and pig hearts without bleeding or complications. “Maria took this polymer platform and turned it into a fixation platform that could be used in many areas in medicine,” Karp recalled.
 Tissium’s platform consists of biocompatible polymers. Image via Tissium.
Tissium’s platform consists of biocompatible polymers. Image via Tissium.
Pharmaceutical executive Christophe Bancel joined after meeting Karp, Pereira, and Langer in 2012 and consulting surgeons across specialties. “I spoke with about 15 surgeons from a range of fields about their challenges,” Bancel said. “All of the surgeons were excited about how the material could impact their practice.” Tissium was founded in 2013 by Pereira, Bancel, Karp, Langer, and others after licensing patents through MIT’s Technology Licensing Office. Pereira later reflected, “The MIT and Harvard ecosystems are at the core of our success. From the get-go, we tried to solve problems that would be meaningful for patients. We weren’t just doing research for the sake of doing research.”
Scaling the material required designing not only the polymer but also surgical accessories. The company partnered with polymer synthesis specialists and developed a method to 3D print casings for polymer-wrapped nerves. “We quickly realized the product is a combination of the polymer and the accessories,” Bancel explained. “It was about how surgeons used the product. We had to design the right accessories for the right procedures.”

Clinical outcomes underscore the demand for alternatives. A meta-analysis of suture-based nerve repairs found only 54 percent of patients achieved highly meaningful recovery. Pereira emphasized that trauma, tension, and misalignment caused by sutures can affect motor function, sensation, and quality of life. By contrast, the company’s atraumatic approach provided consistent recovery in trials. Six additional products are in development, including one in a hernia clinical trial and another set to begin in cardiovascular surgery.
Manufacturing on Demand
“Not only can this be used for tissue fixation broadly across medicine, but we can leverage the 3D printing method to make all kinds of implantable medical devices from the same polymeric platform,” Karp said. Bancel described the FDA clearance as the start of a new phase. “Now it’s our responsibility to show this works with other applications and can benefit more patients.”

“It’s the best possible outcome for your research to generate not just a paper, but a treatment with potential to improve the standard of care along with patients’ lives,” Karp said. Langer added, “It’s wonderful to see the research we started at MIT reach the point of FDA approval and change people’s lives.”
You might also like:
UC San Diego Health Performs World’s First Spine Surgery with Fully Customized 3D Printed Implant: This innovation offers particular promise for patients with spinal stenosis, degenerative disc disease, or spinal deformities, who could benefit from a level of customization previously unavailable in spine surgery.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Anyer Tenorio Lara

Leave A Comment