3D LifePrints’ EmbedMed platform achieves FDA 510(k) clearance Medical
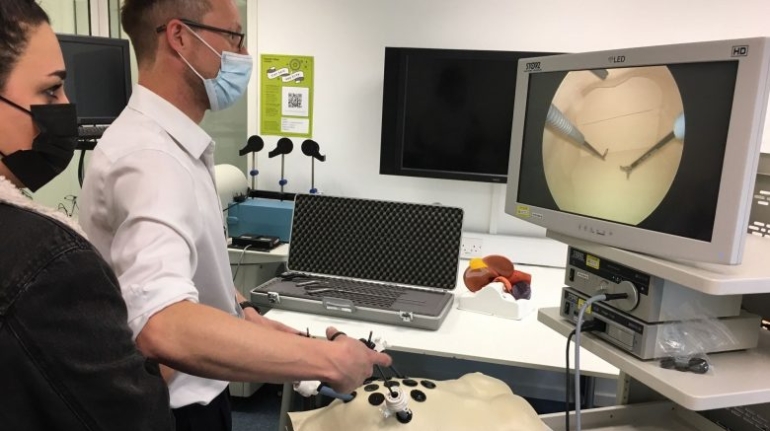
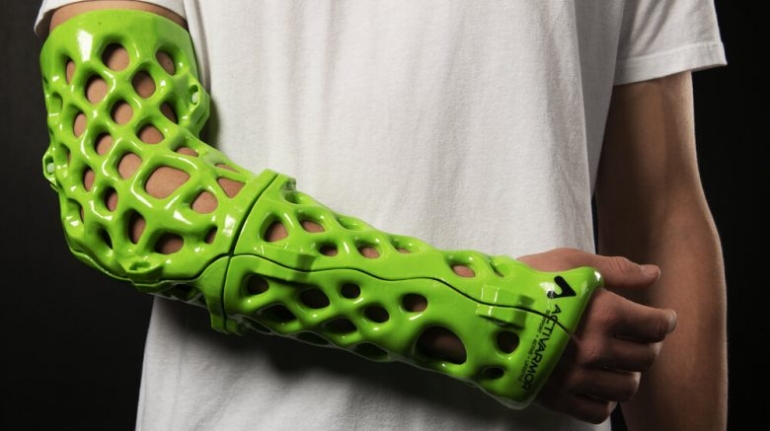
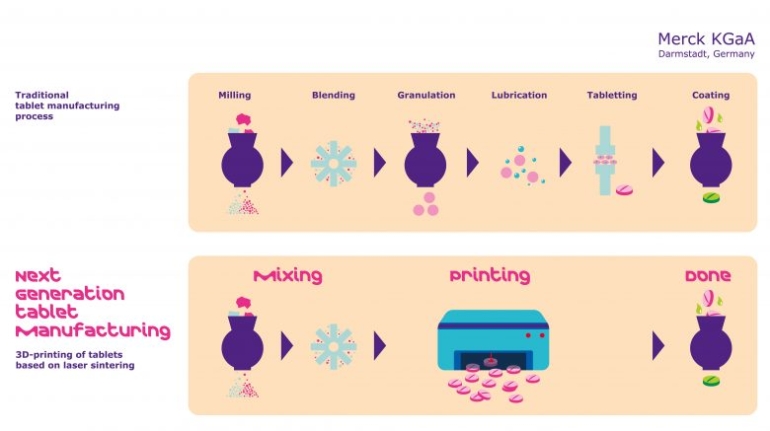
3D LifePrints, a leading provider of personalized surgery that has supplied thousands of patient-specific devices in the UK and Europe, and more recently in the US, has received FDA 510(k) clearance for the company’s cranio-maxillofacial products and services, which are included in the EmbedMed platform. This clearance follows the company’s ISO 13485 medical certification in 2021.