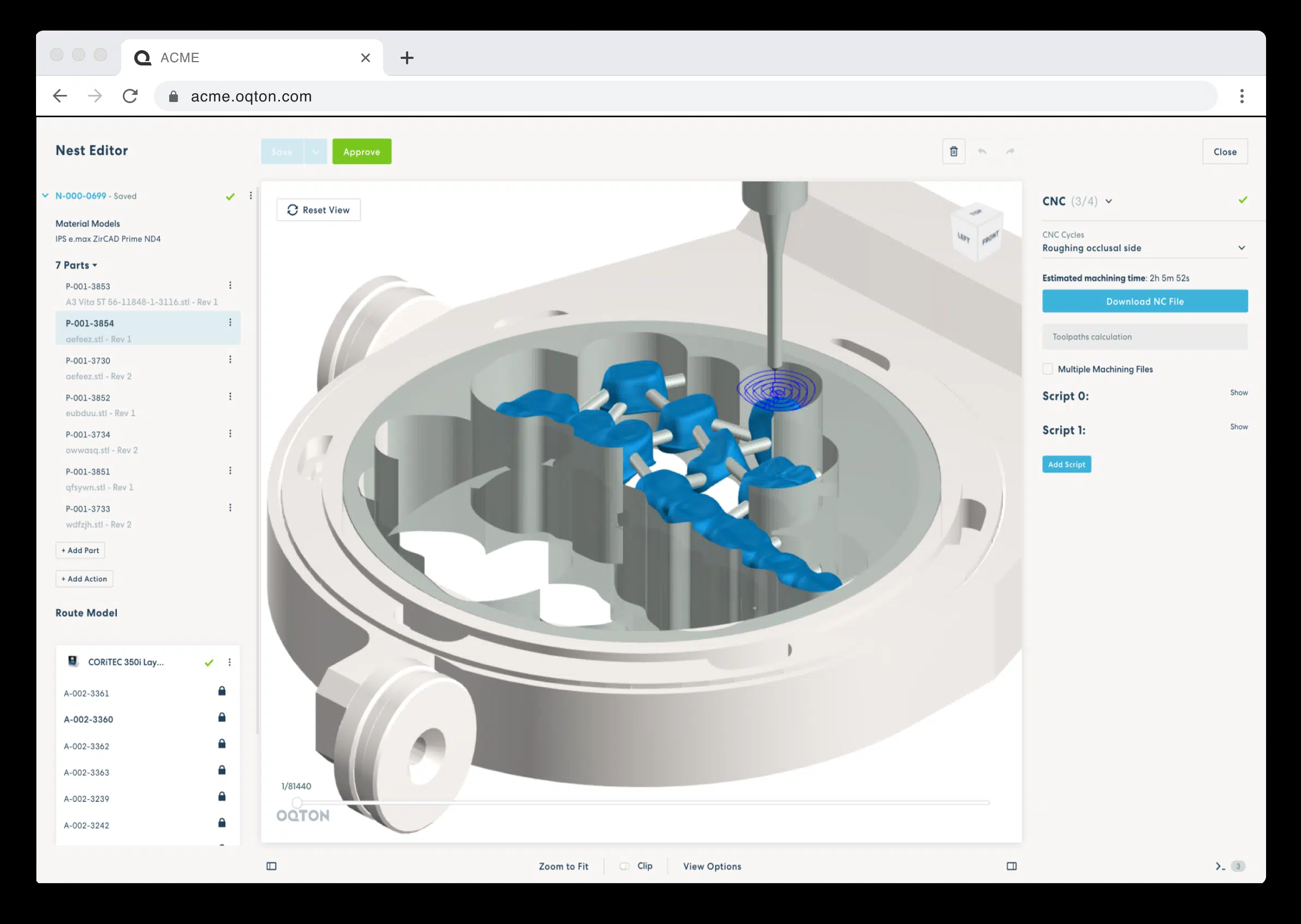
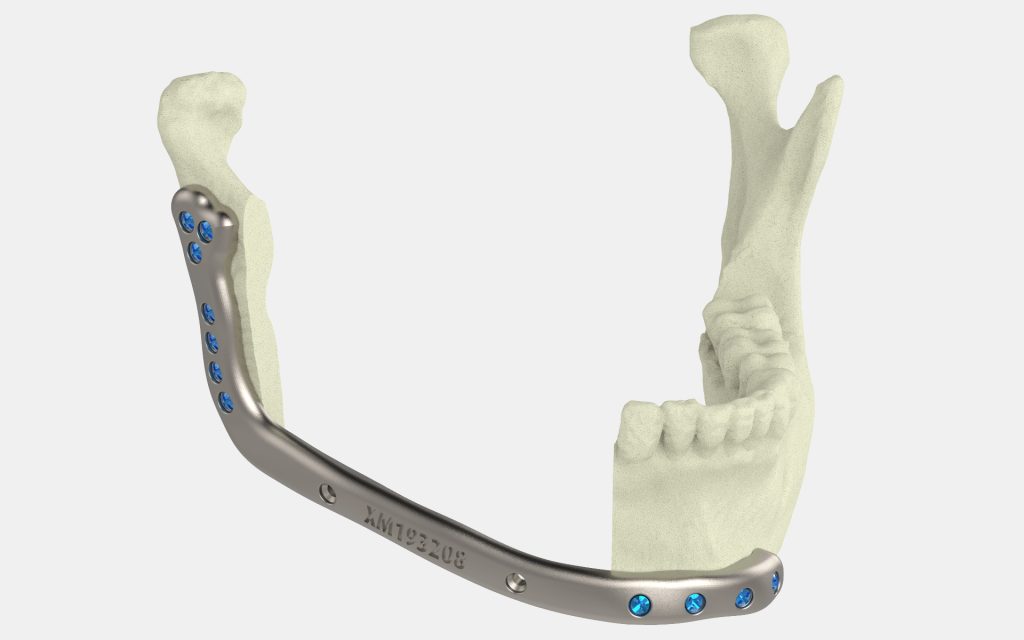
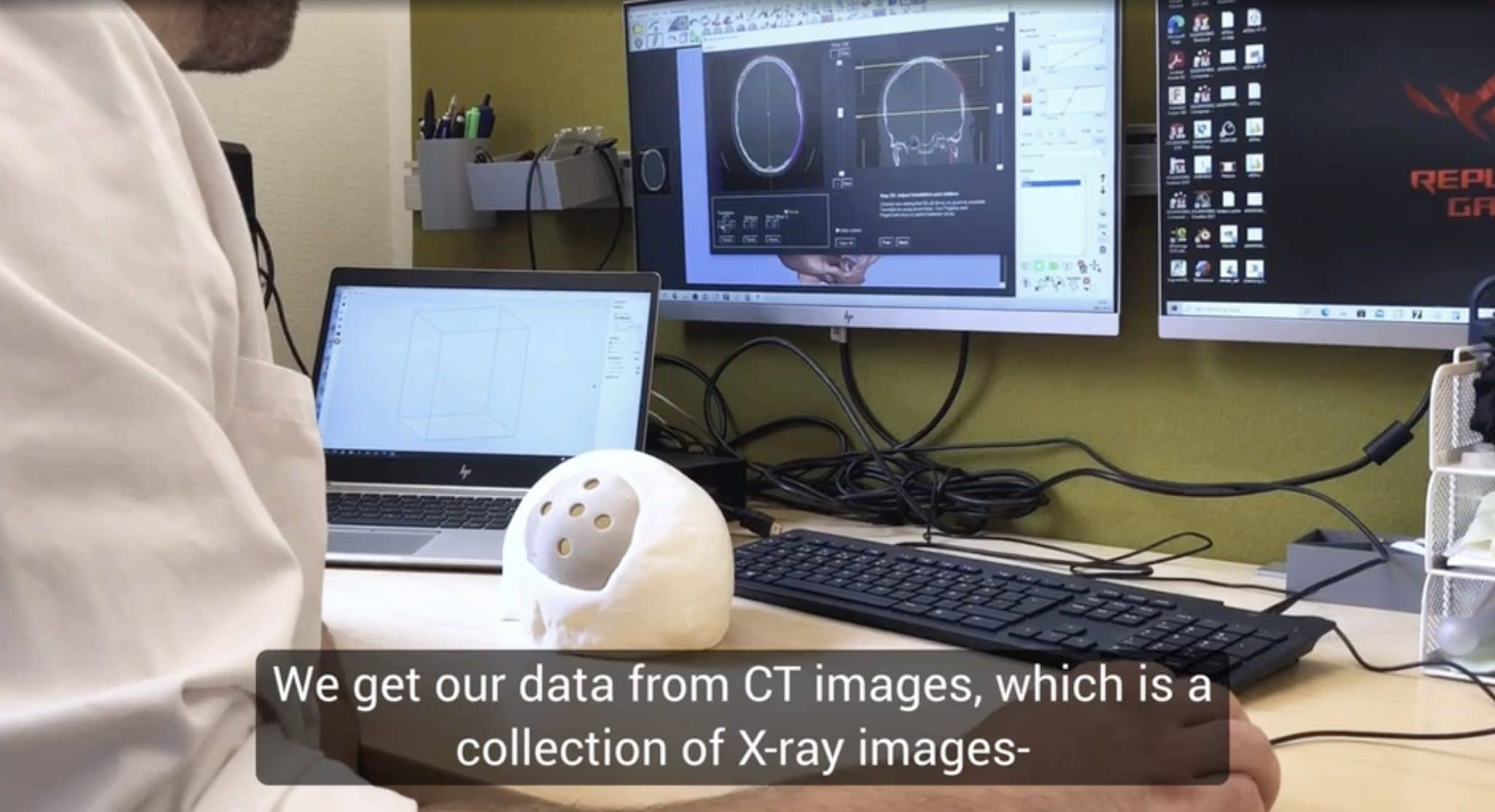
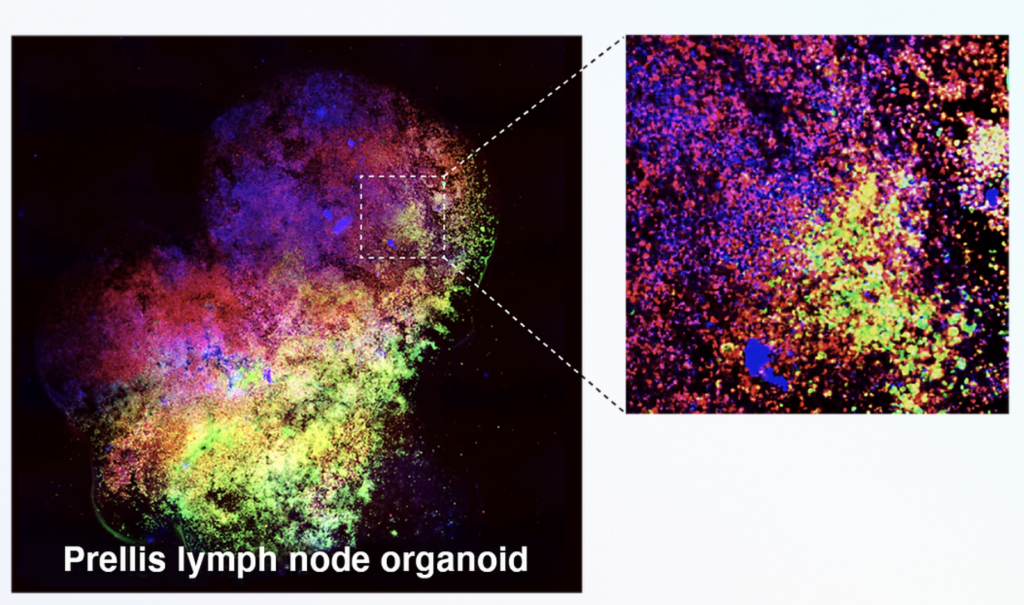
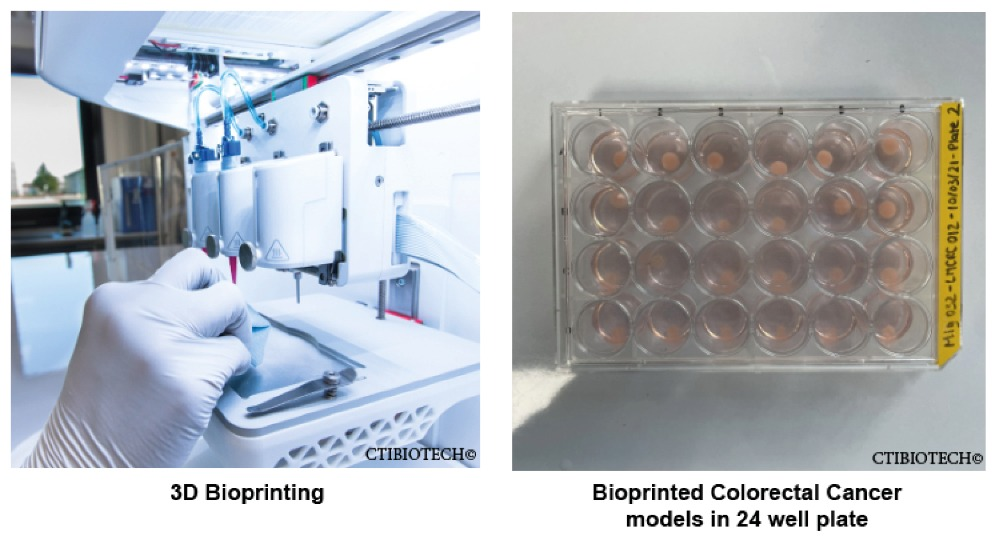
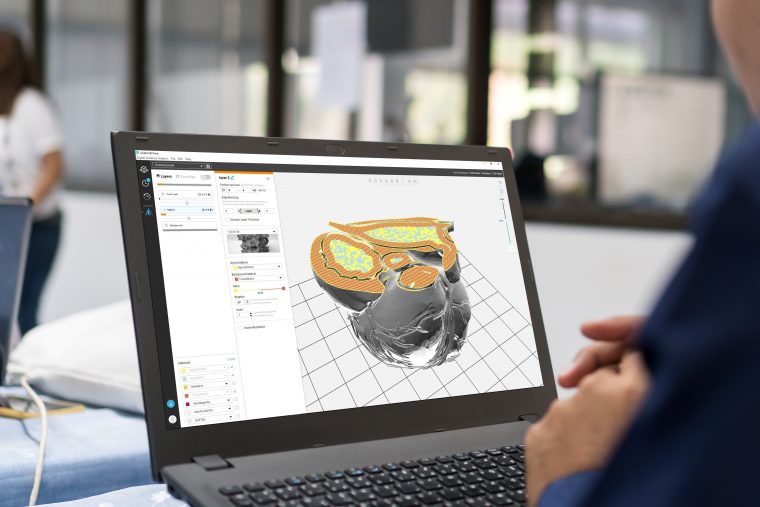
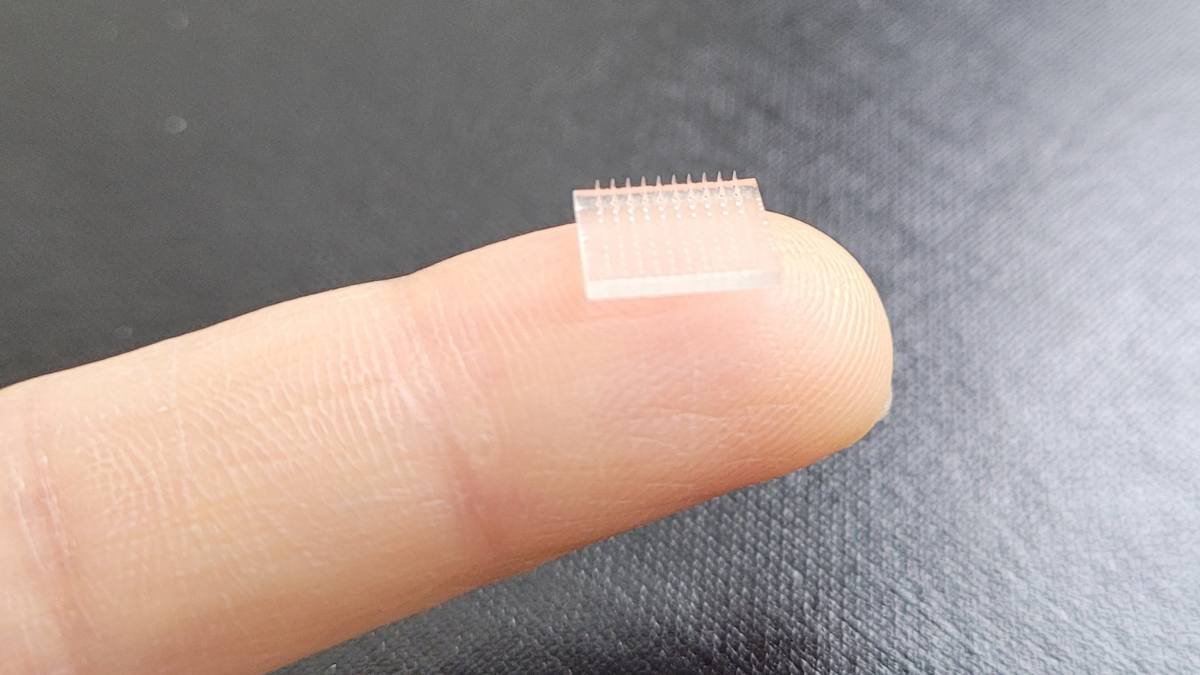
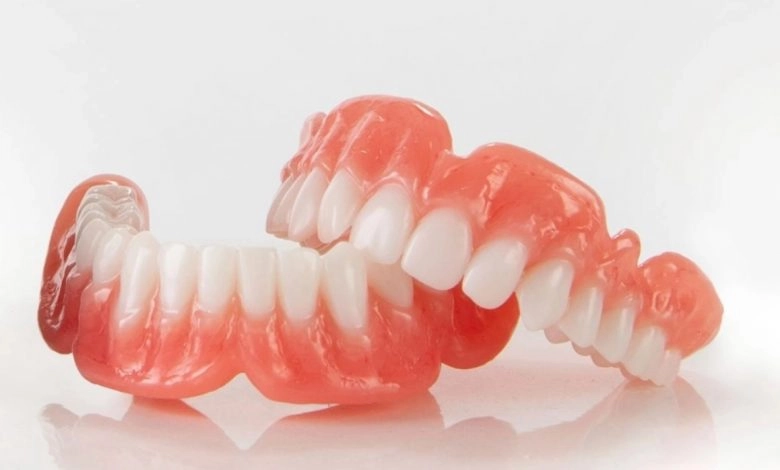
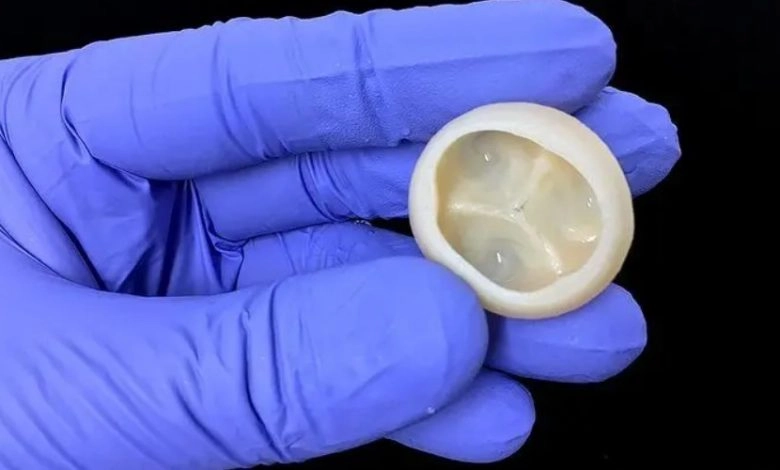
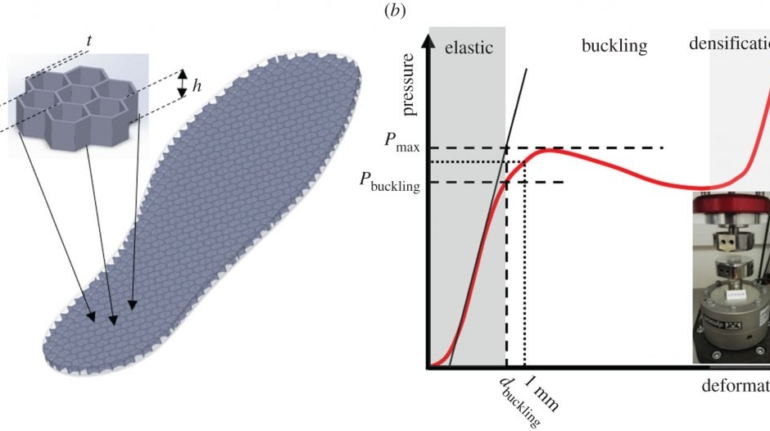
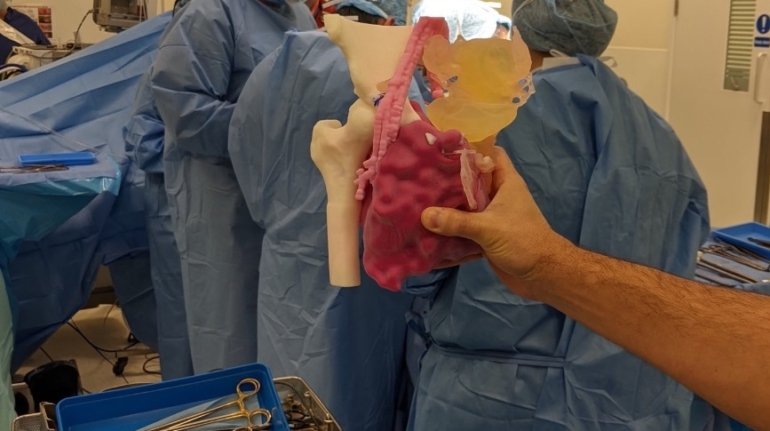
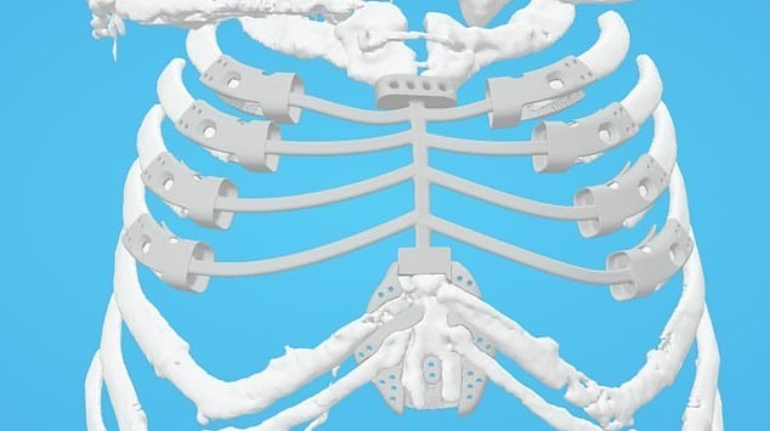
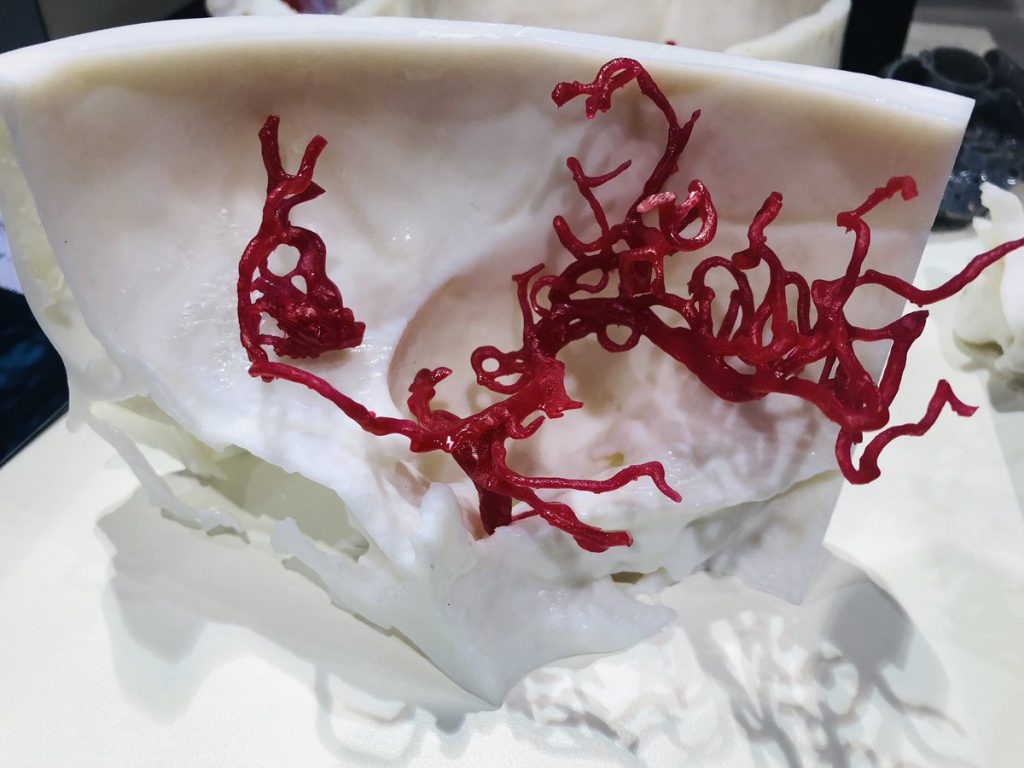
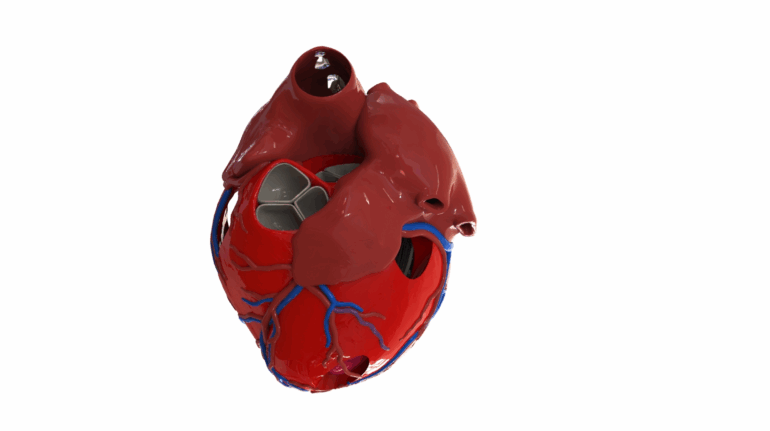
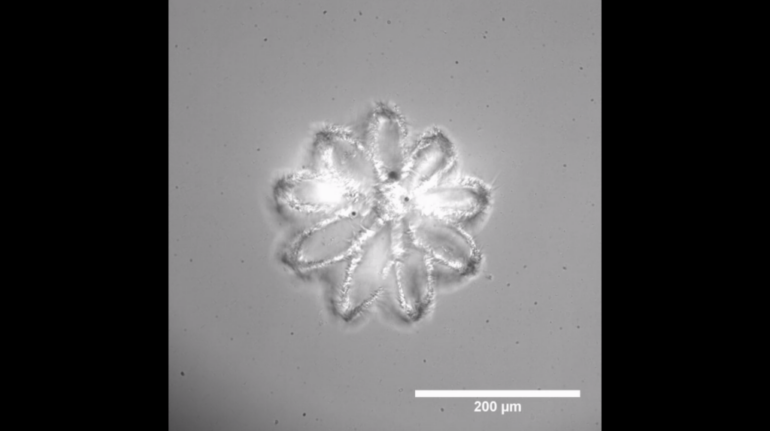
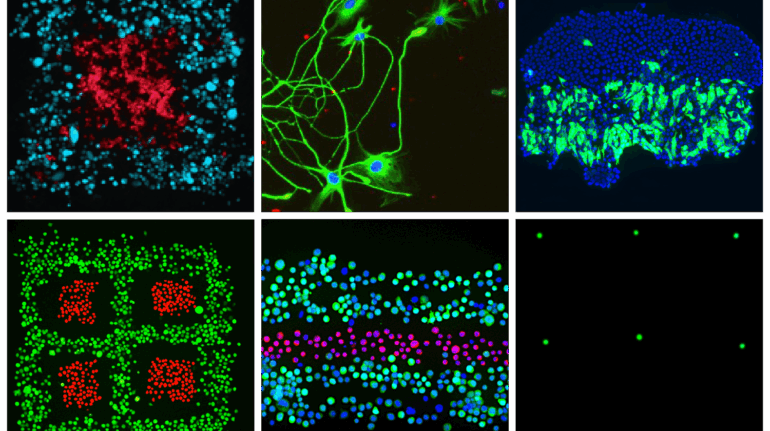
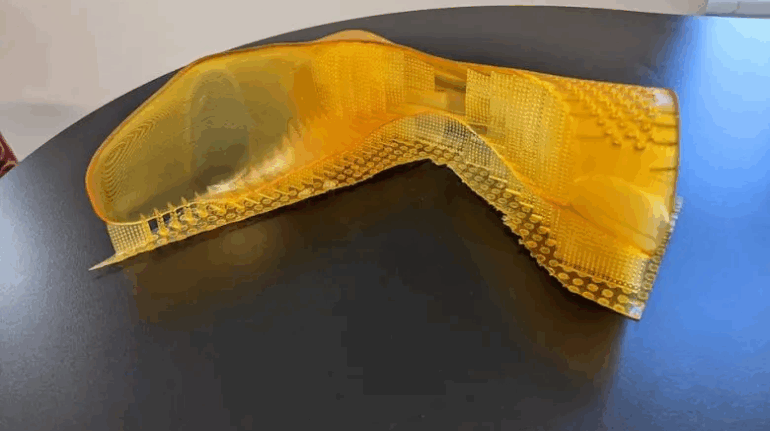
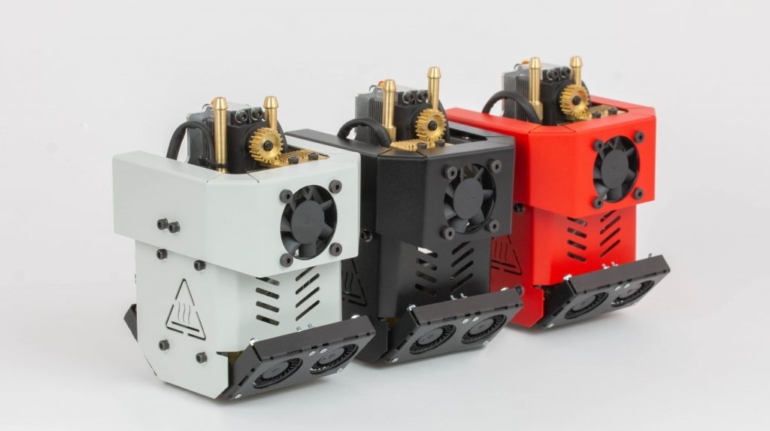
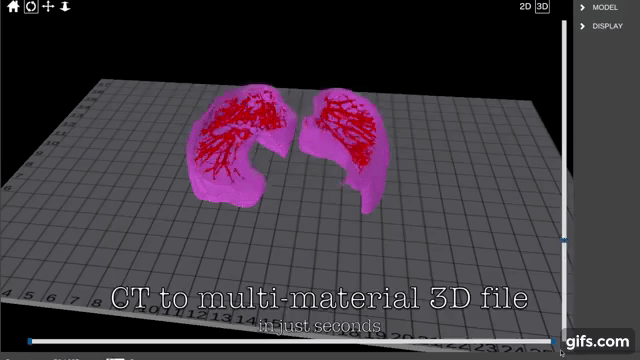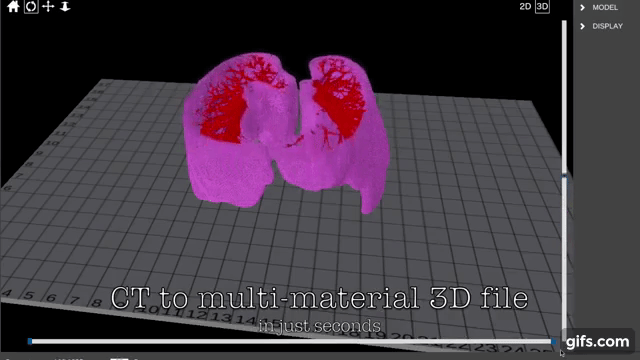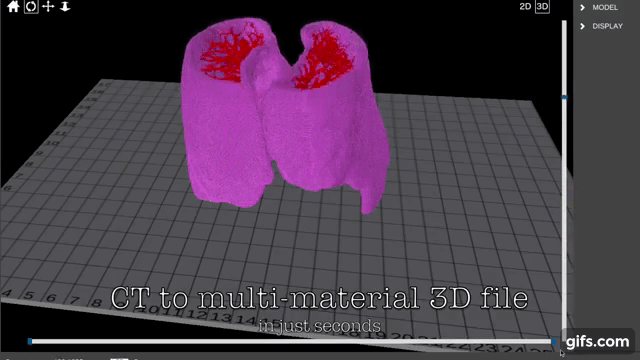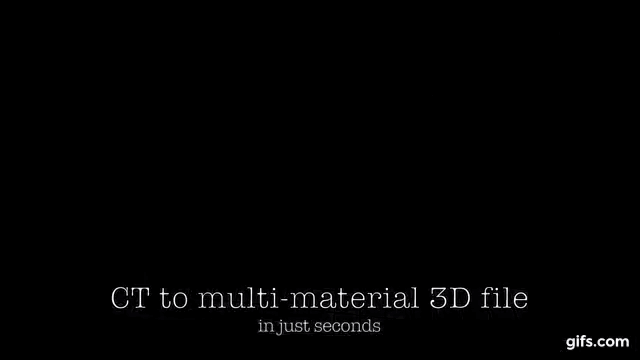
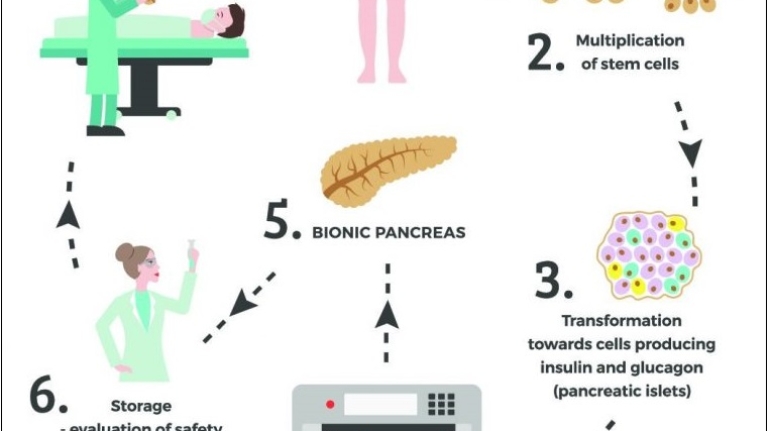
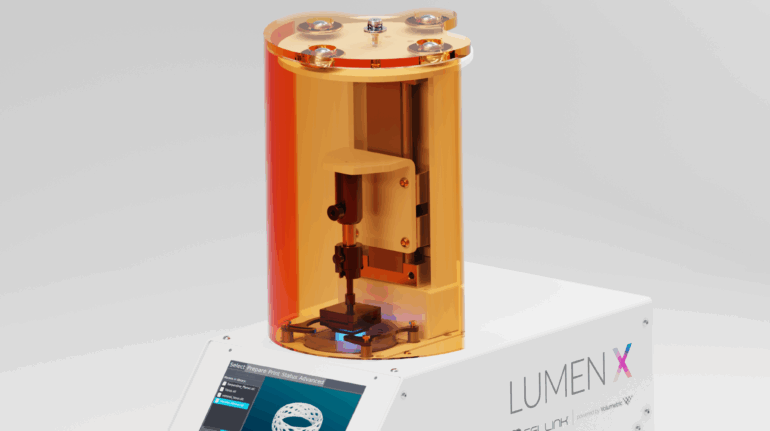
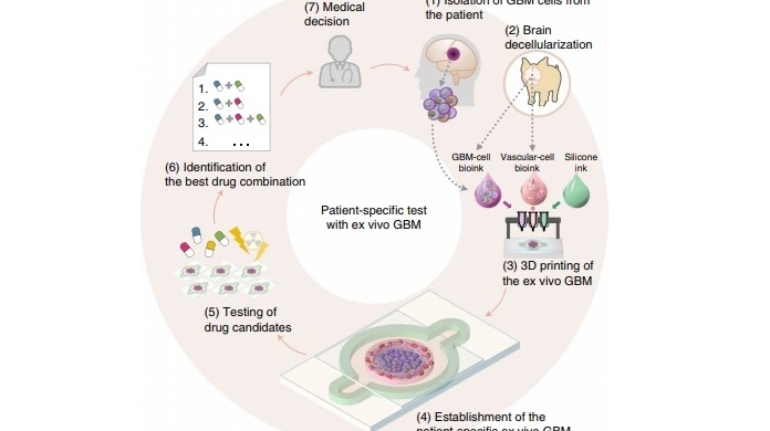
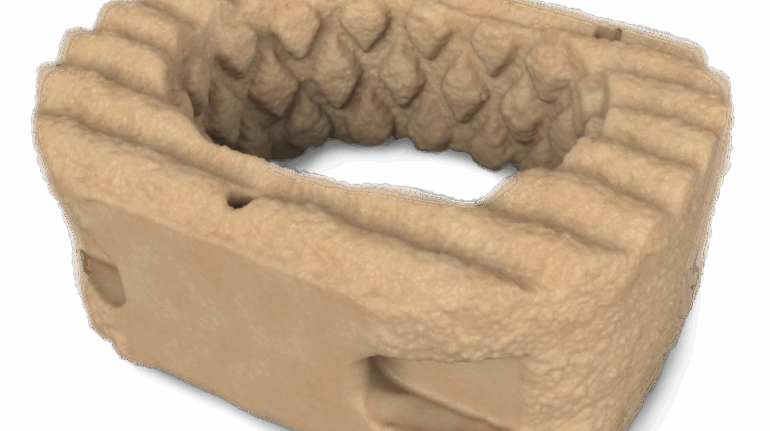
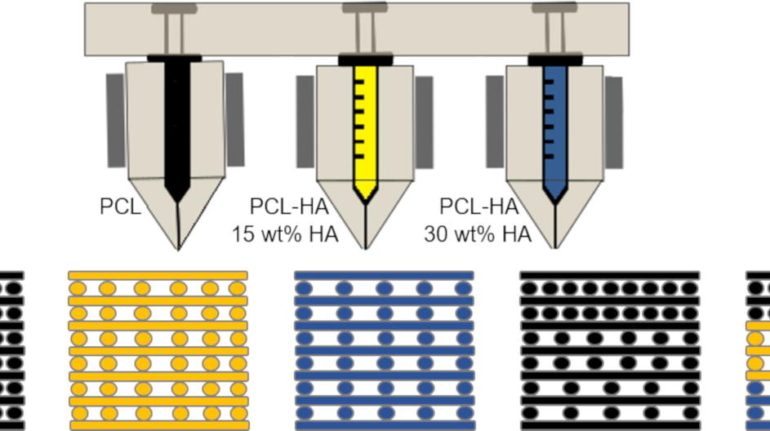
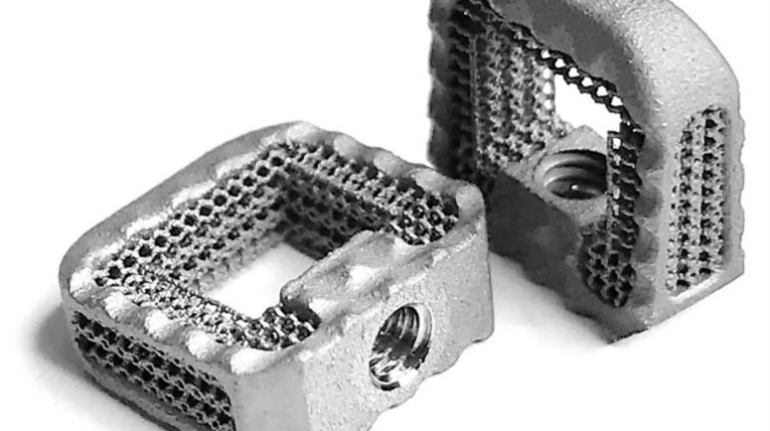
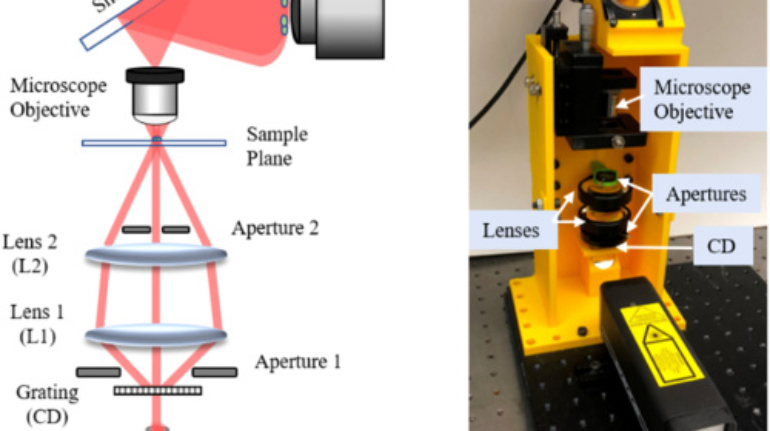
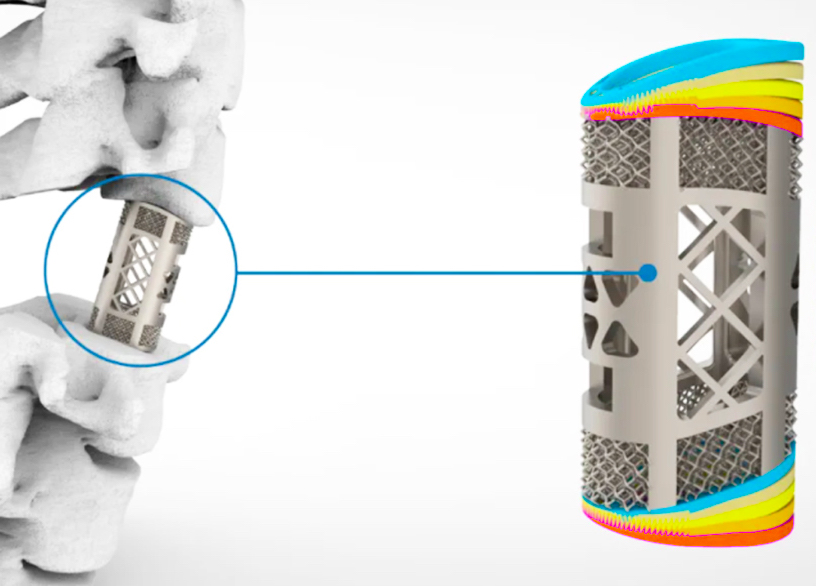
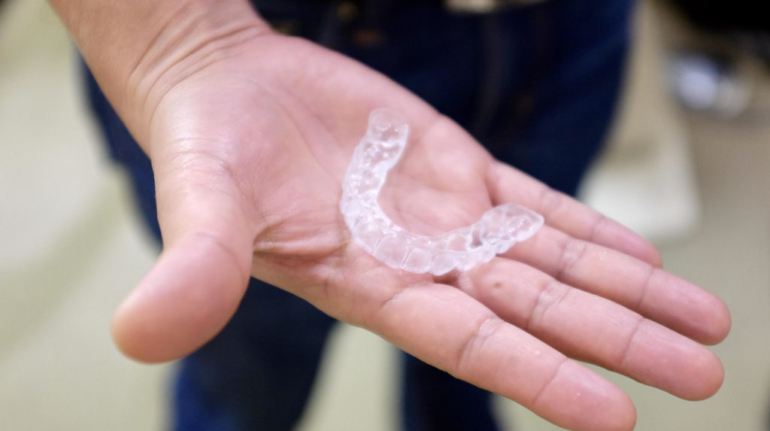
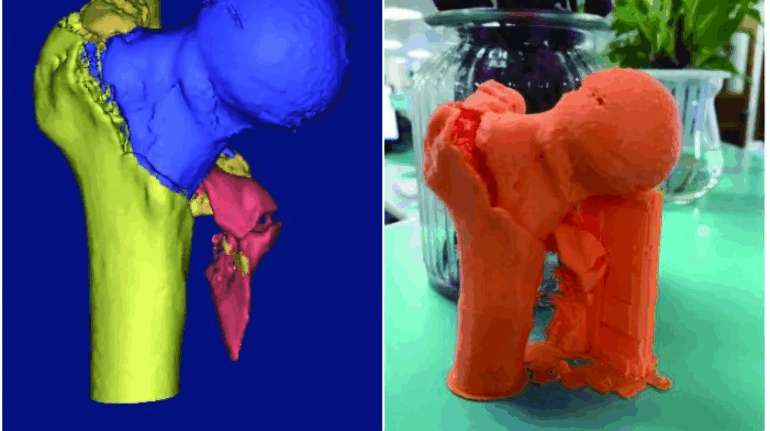
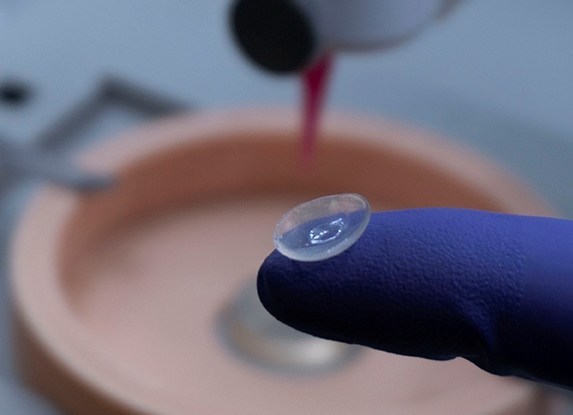
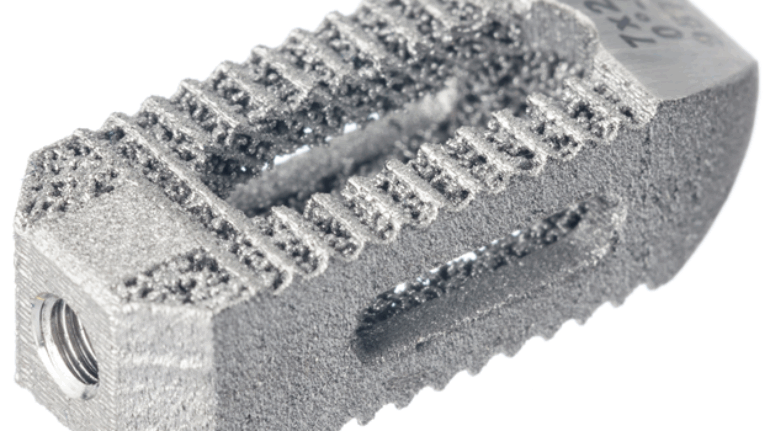
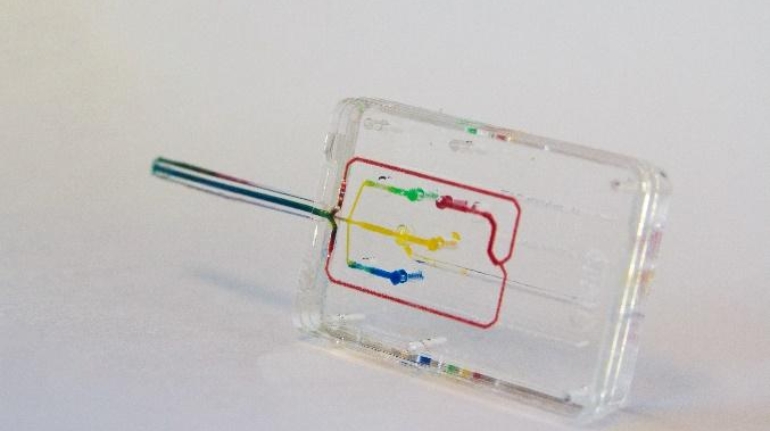
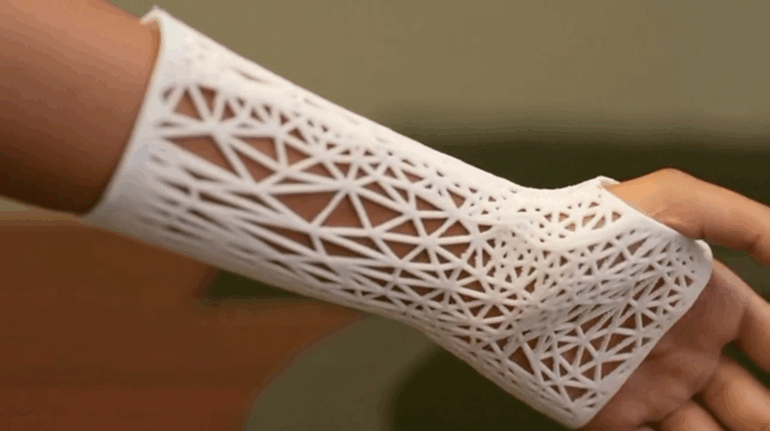
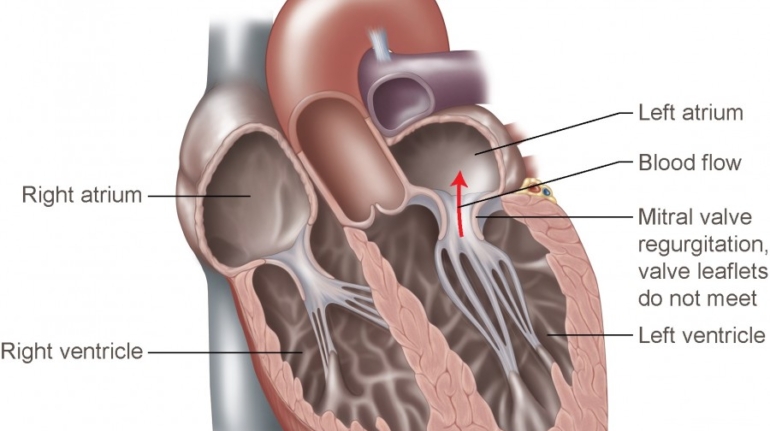
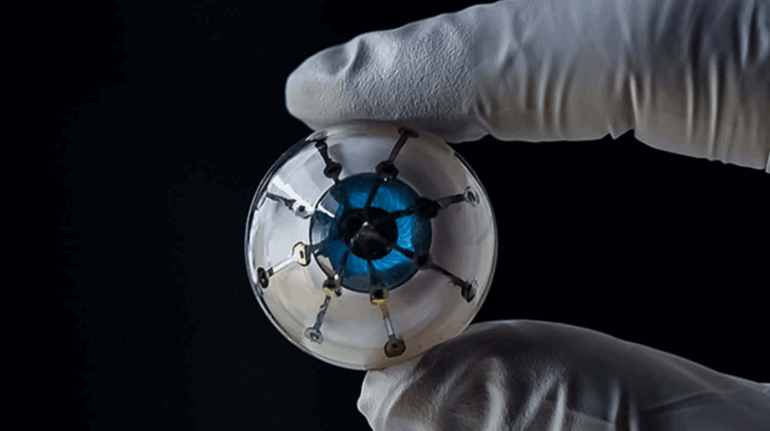
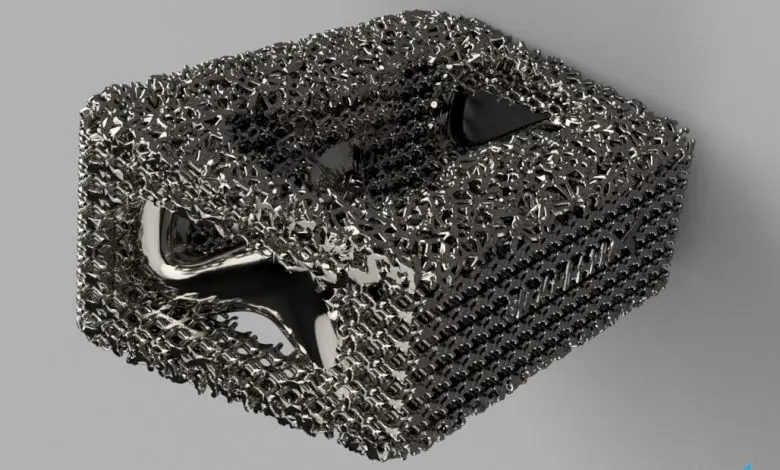
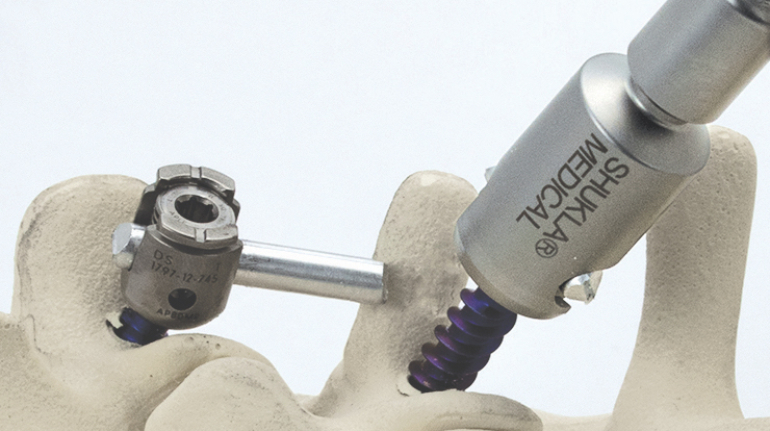
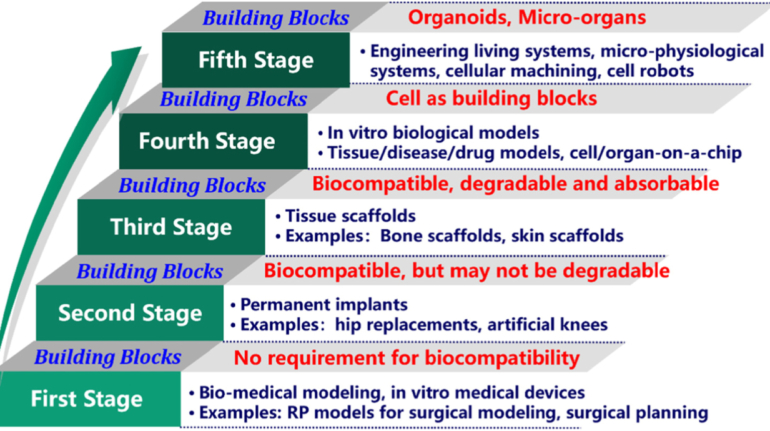
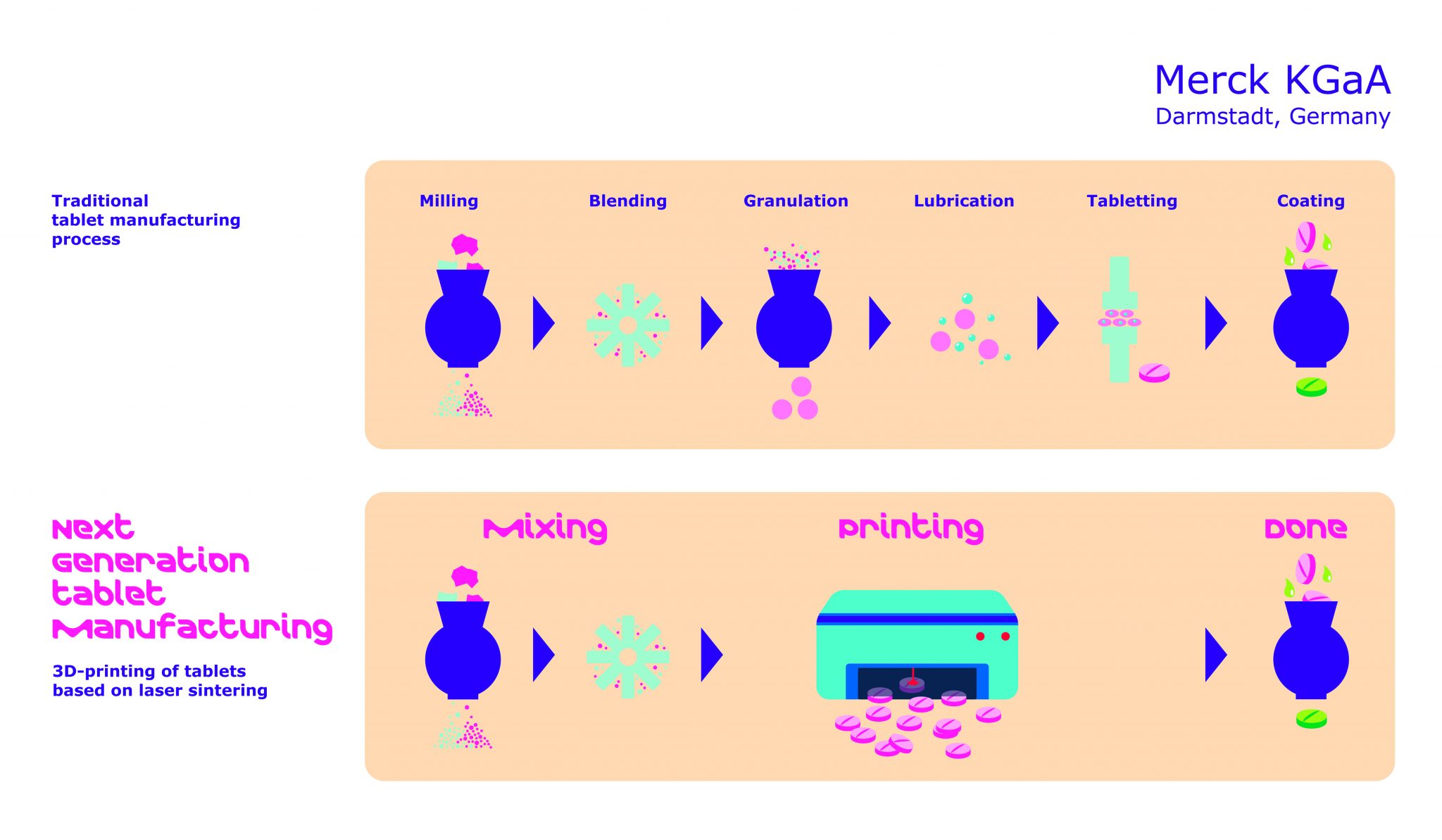
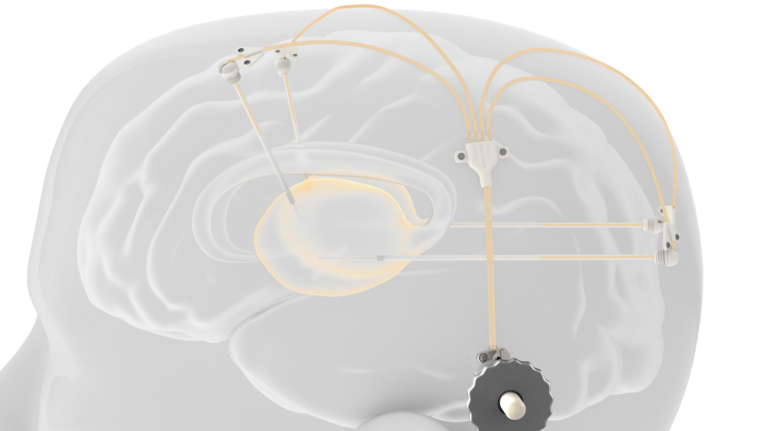
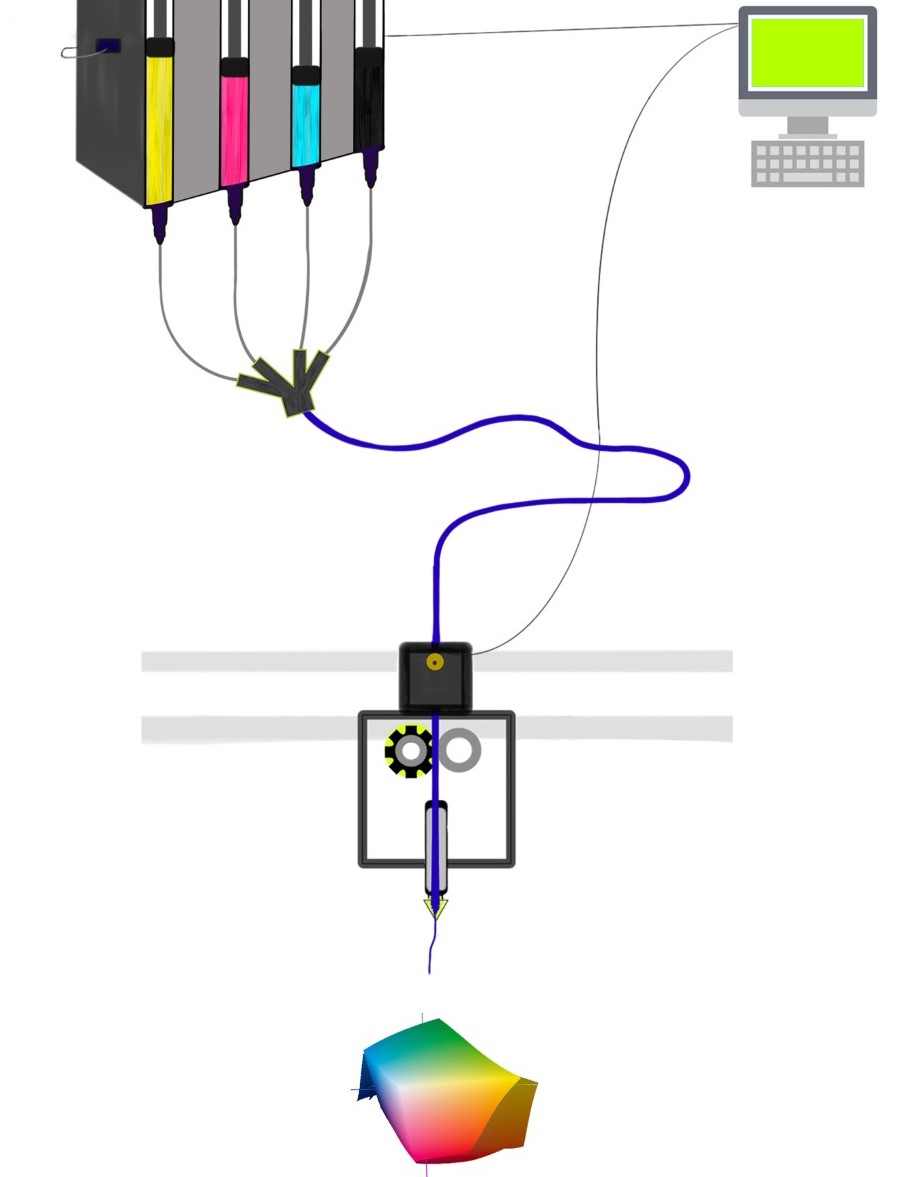
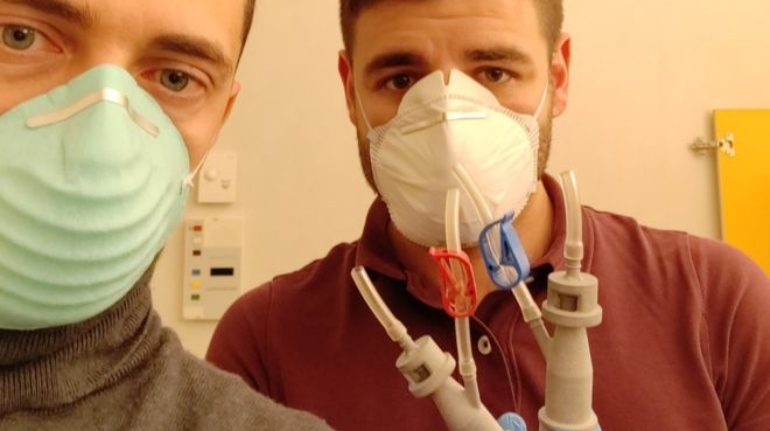
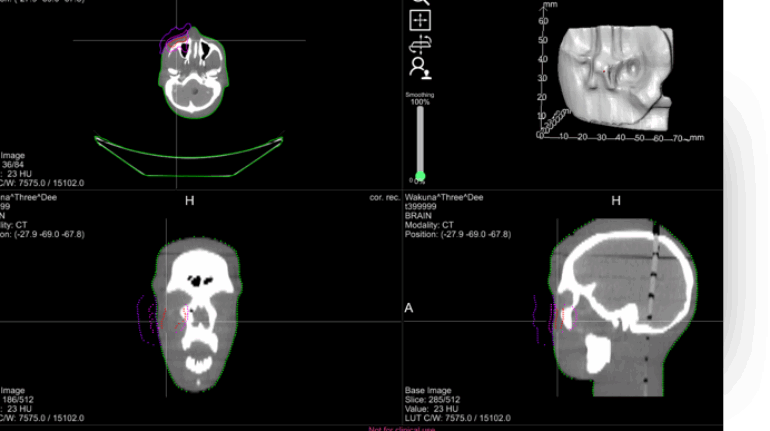
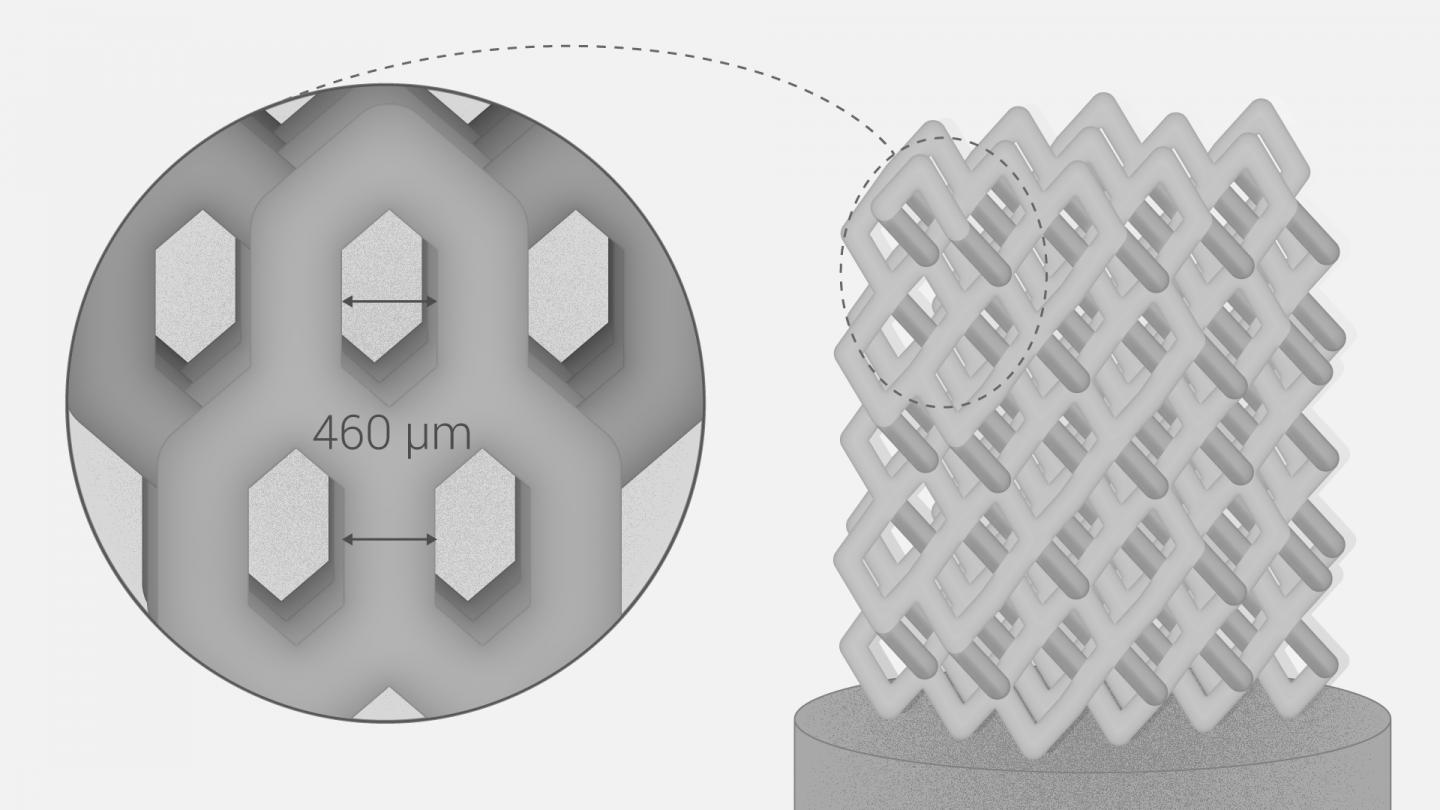
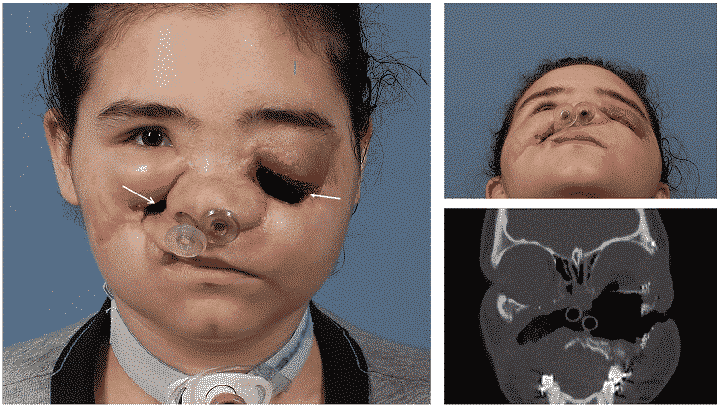
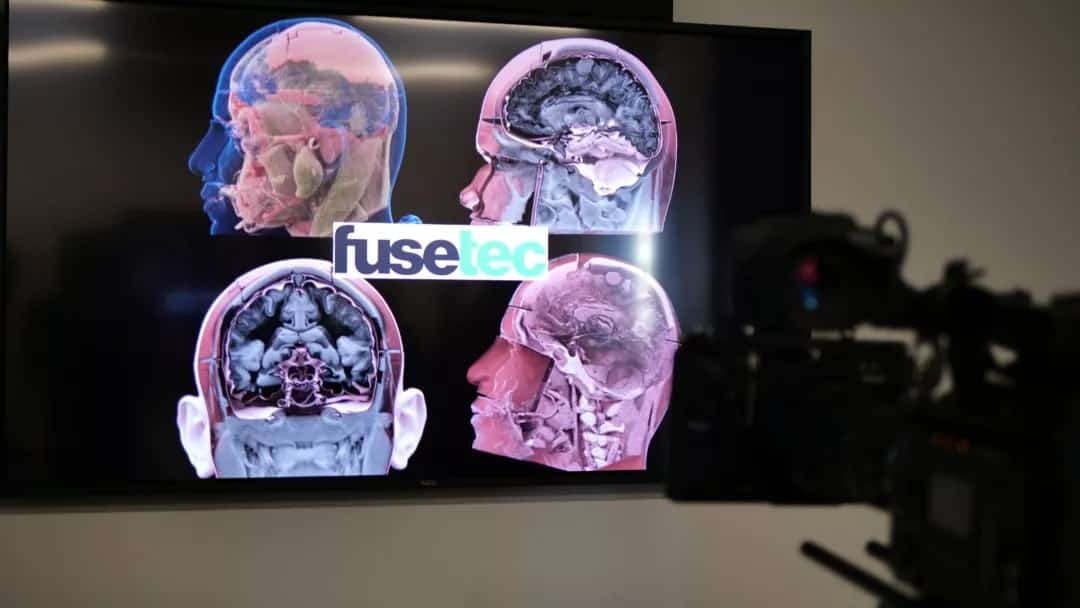
Formlabs, 3D Systems and Xact Metal Announce Dental 3D Printing Updates Ahead of Lab Day 2022
A flurry of dental 3D printing developments have been announced ahead of the start of America’s largest dental laboratory event, Lab Day 2022, tomorrow in Chicago.