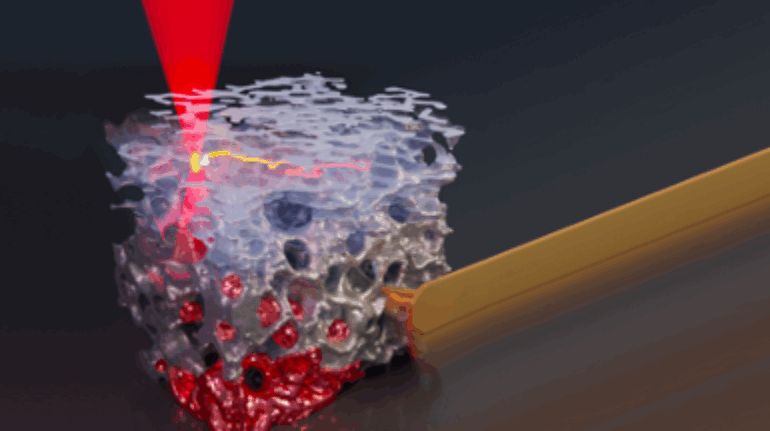
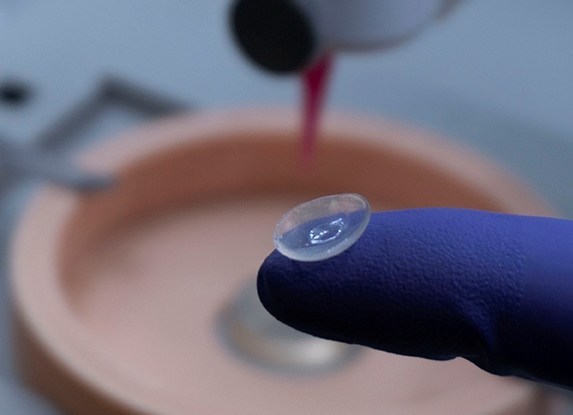
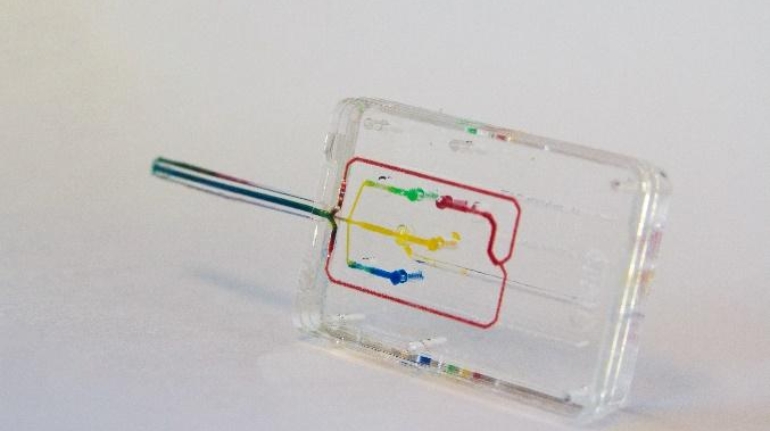
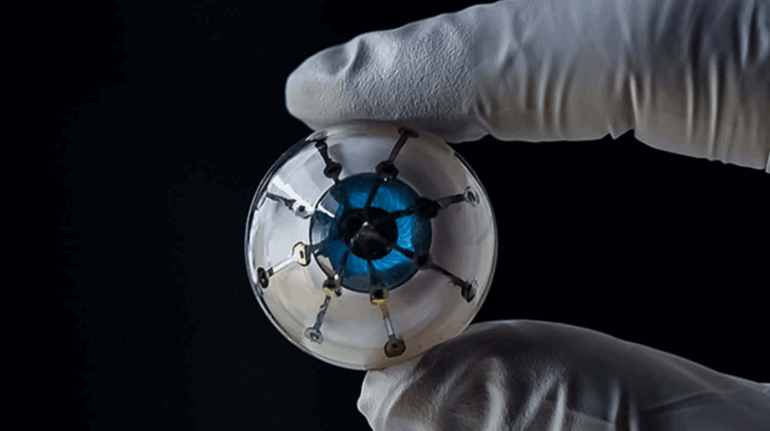
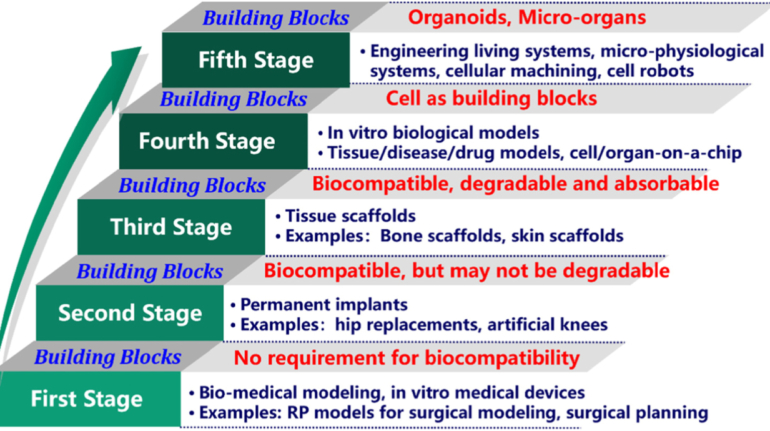
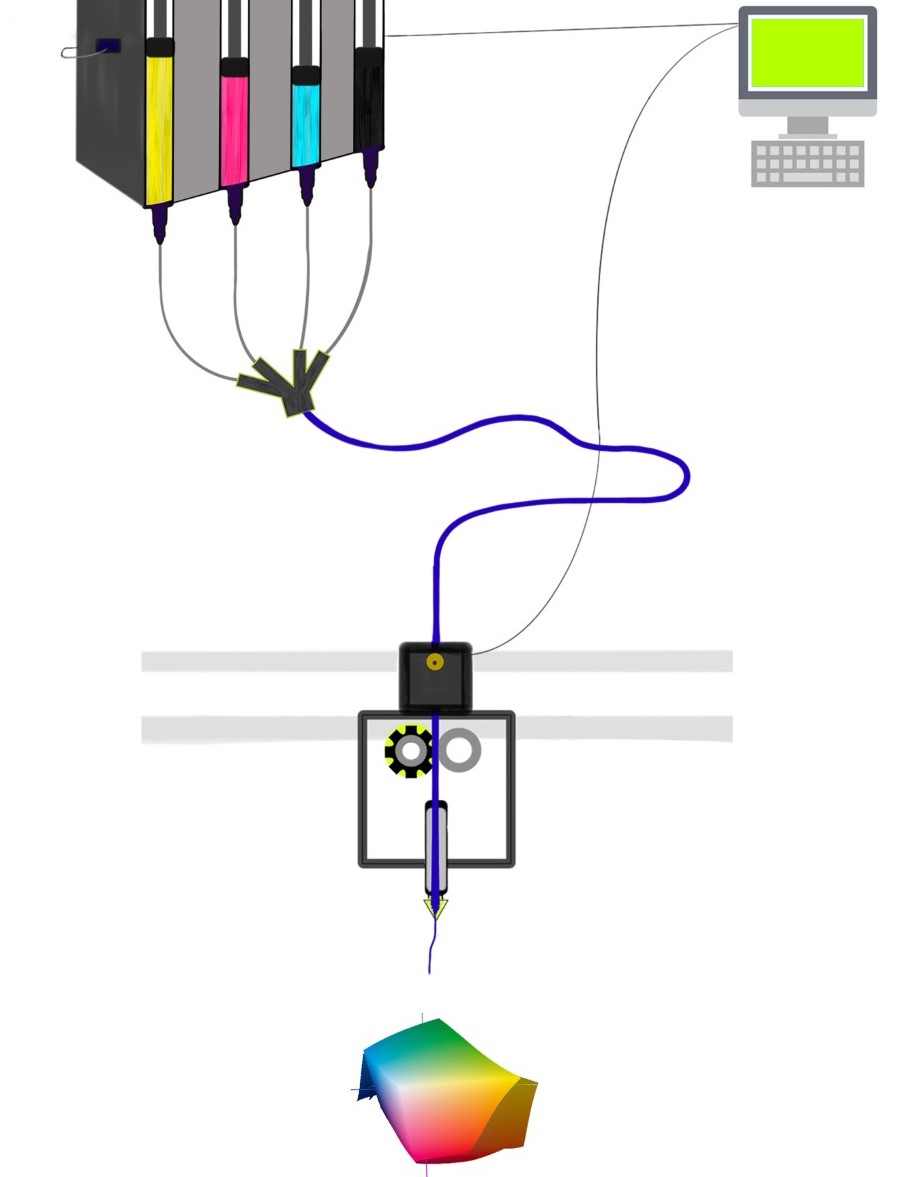
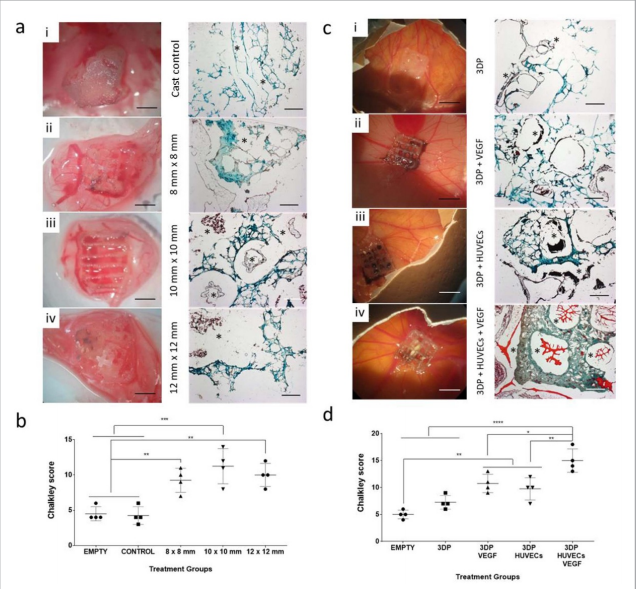
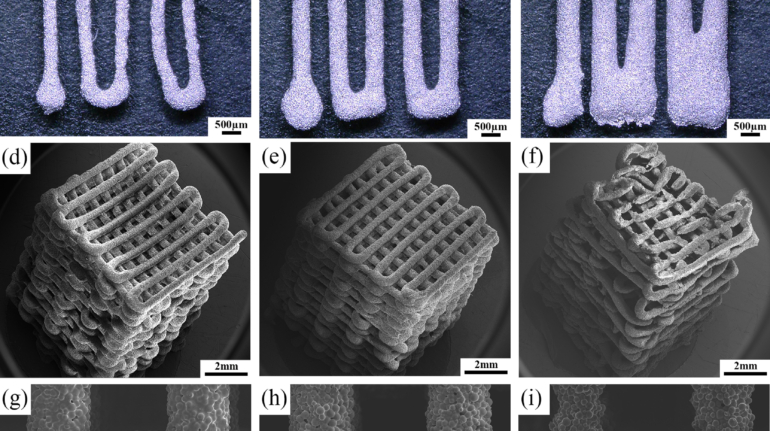
Brinter Launches New Visco Bio Printing Heads with Puredyne Bioprinting
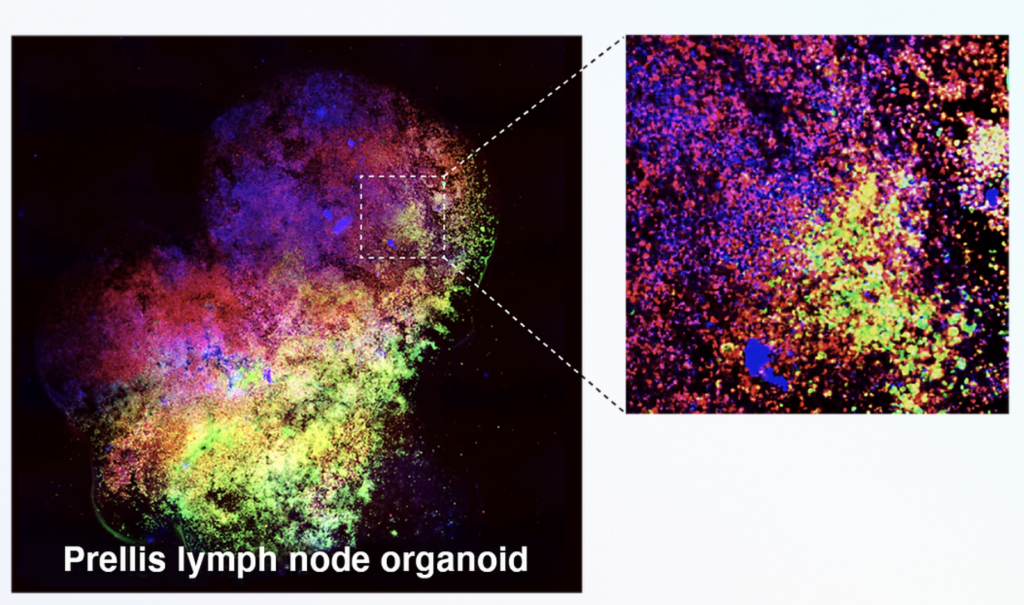
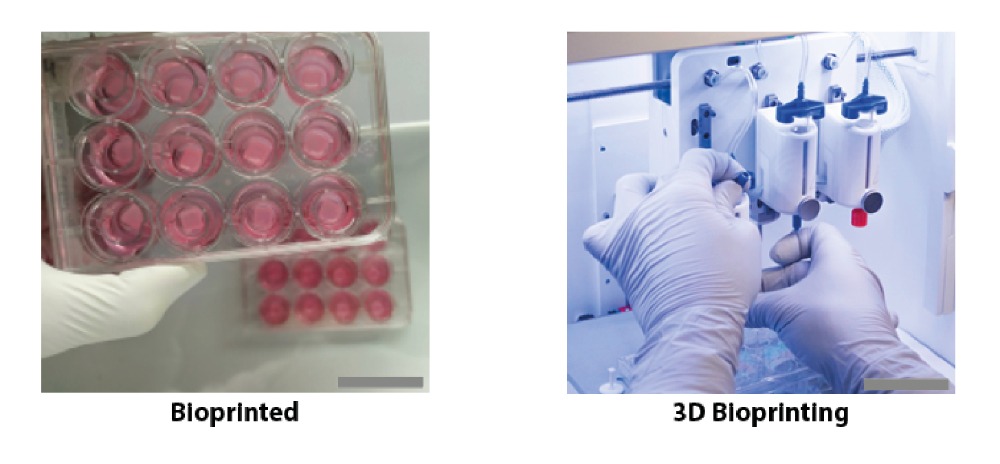
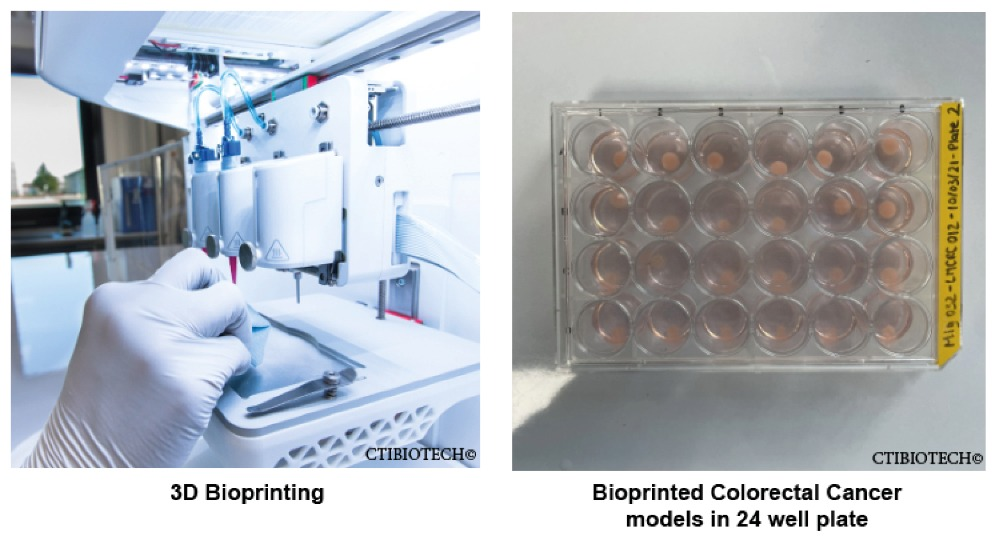
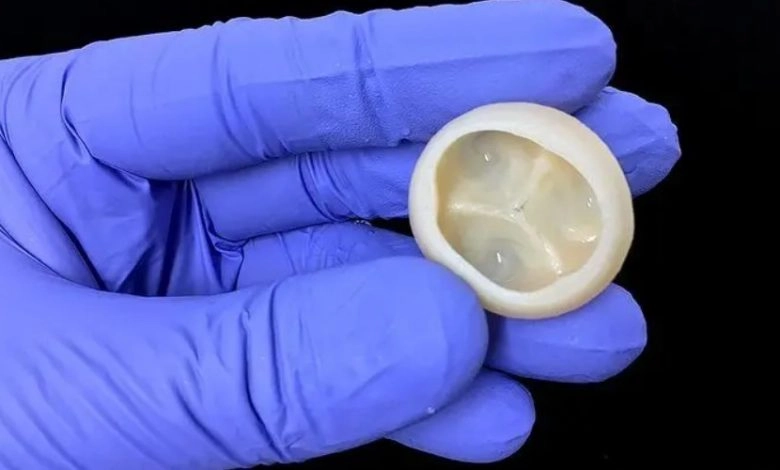
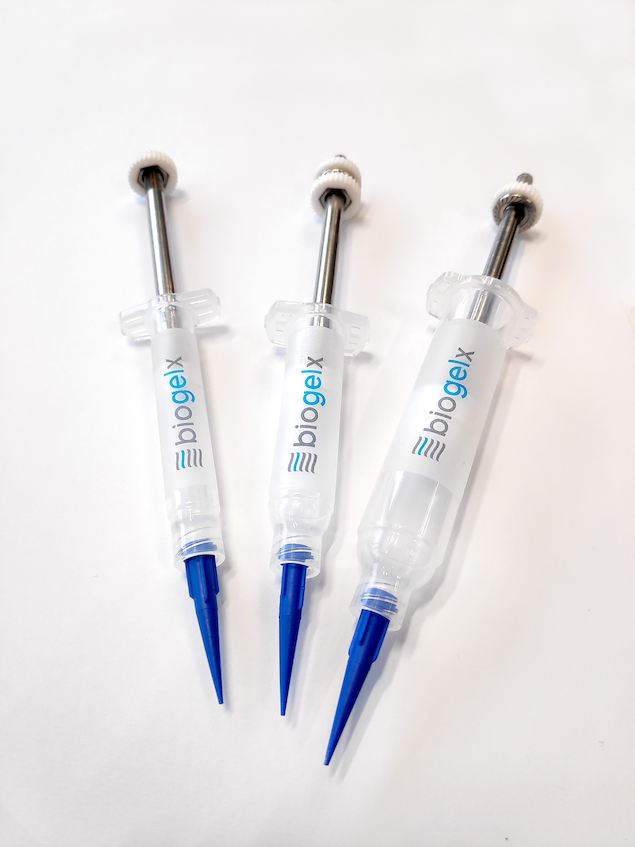
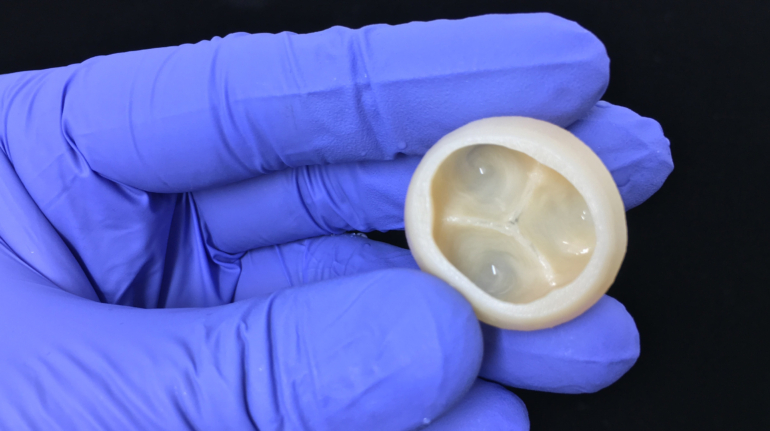
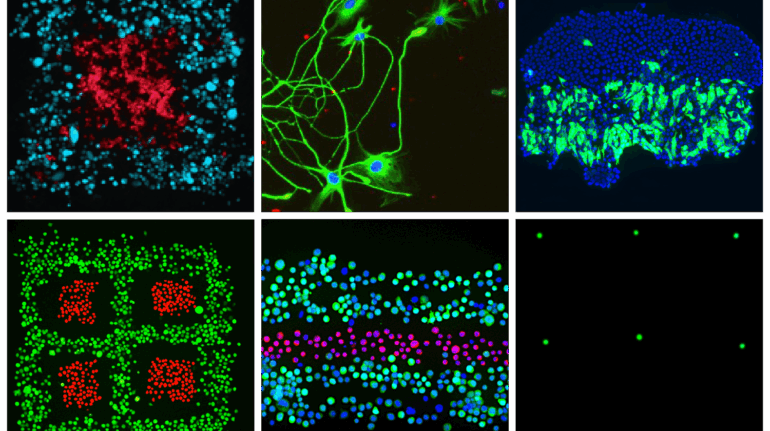
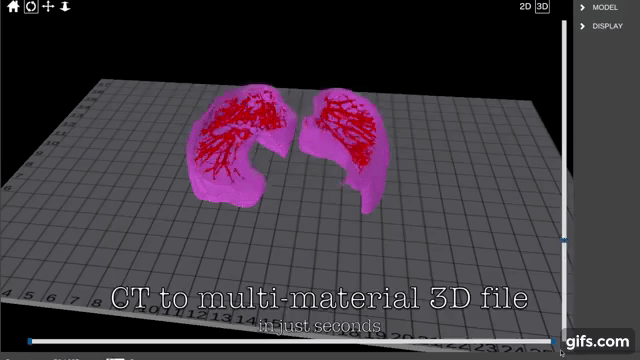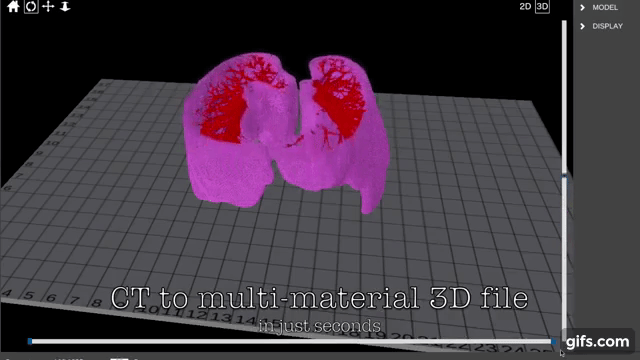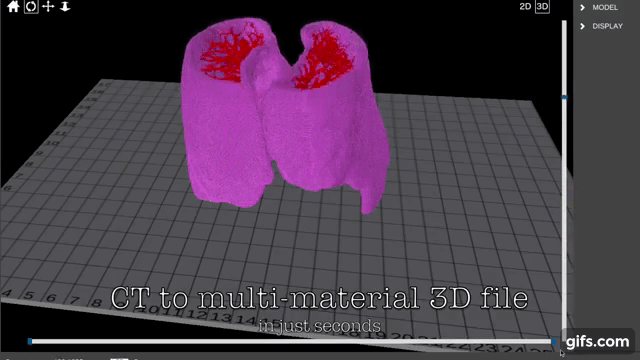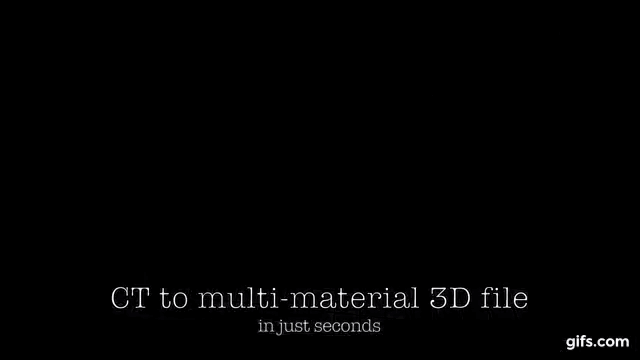
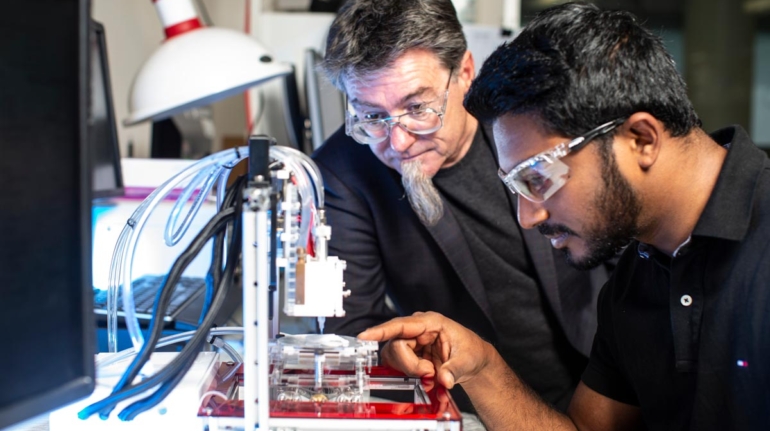
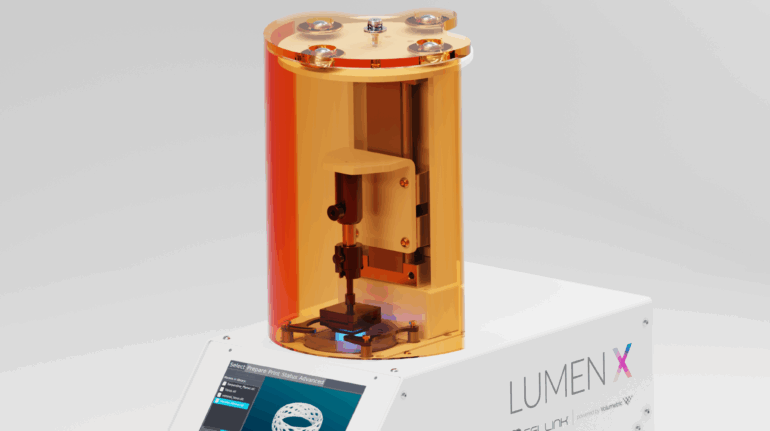
Finnish bioprinting startup Brinter released the new extrusion-based Visco Bio medical print head for its Brinter bioprinters. The new modular print heads enable nearly zero dead volume, meaning that expensive medical grade material waste is cut to a minimal level. The Finnish company continues to develop and implement new features to its device on the back of a strong first year’s growth year in 2021.