A cornea 3D printed at India’s L V Prasad Eye Institute (LVPEI), Indian Institute of Technology (IIT) Hyderabad and Centre for Cellular and Molecular Biology has been successfully animal tested for the first time.
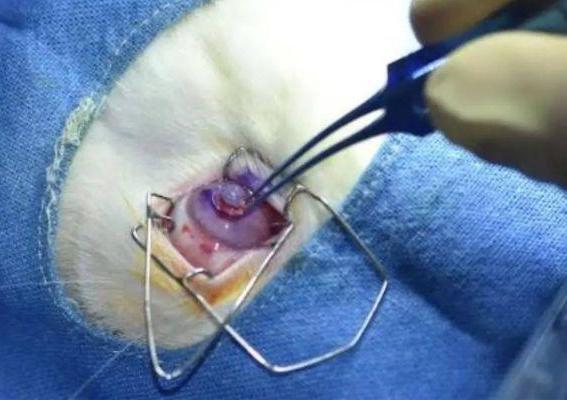
Made from a bio-ink derived from human donor tissues, the artificial material and animal residue-free cornea was transplanted into the eye of a rabbit. Due to its “completely natural” base, the implant’s developers say it could soon be “used in human beings,” to treat corneal scarring as well as other serious eye conditions that can cause blindness.
“It is the first 3D printed human cornea that is optically and physically suitable for transplantation,” said Dr Sayan Basu and Dr Vivken Singh, Lead Researchers at LVPEI. “The bio-ink used to make this 3D printed cornea can be sight-saving for army personnel at the site of injury to seal corneal perforation and prevent infection during war-related injuries or in a remote area with no tertiary eye care facility.”
Why bother bioprinting corneas?
While the cornea, the transparent part of the eye that covers the iris and pupil, is generally resilient and capable of healing from minor abrasions, anything from not using contact lenses properly to contact injuries can cause it to scar. Even those living healthy lifestyles that take great care of their eyes, can fall victim to issues like corneal dystrophies, which cause clouded vision, and often run in families.
According to the USA’s National Eye Institute, the main remedies used to treat corneal conditions are still laser therapy, or the transplant of either organic or artificial tissues. However, while prosthetics don’t restore patients’ sight, cell-based transplants tend to be sourced from animals in a way that can make them a rejection risk, and minimize their application among religious communities.
“Although corneal substitutes are being actively researched throughout the world, they are either animal-based or synthetic,” explain the researchers. “Pig or animal-based products are unsuitable for India and major parts of the developing world because of issues related to social and religious responsibility.”
India’s animal testing breakthrough
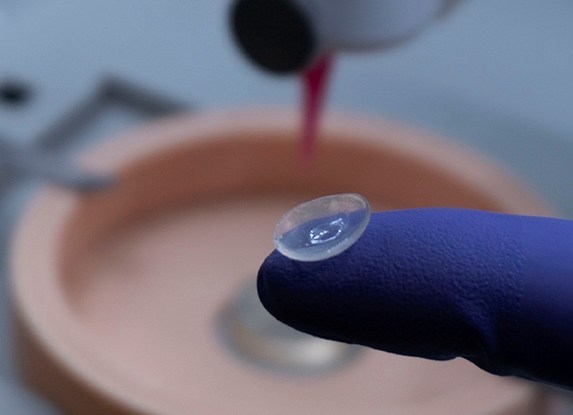
To get around some of the cultural and religious issues surrounding the use of certain animal cells in ocular implants, the researchers have based theirs around human donor tissues instead. Specifically, using a decellularized corneal tissue matrix and stem cells extracted from the human eye, the team have developed a biometric hydrogel.
Completely free of synthetic components, the material is designed to provide the ideal microenvironment for stromal regeneration. In fact, Dr Falguni Pati, an Associate Professor at IIT-Hyderabad, says the bio-ink is even capable of doing so while maintaining the curvature and thickness of the bioprinted cornea,” thus facilitating its implantation.
Manufacturing on Demand
Using their unique material, the scientists have created what’s said to be India’s first implant of its kind, before transplanting it into the eye of an animal test subject. With the surgery said to have gone well, LVPEI’s Dr Sayan Basu and Dr Vivken Singh say the bioprint could soon prove useful for treating ocular scarring or conditions like keratoconus, where the cornea bulges, causing considerable vision problems.
As with the rest of the implant’s R&D, the translational studies designed to prepare it for clinical trials are being funded by philanthropists. Moving forwards, with the backing of the Sree Padmavathi Venkateswara Foundation, a group set up to support promising Indian medical research, it is hoped the technology will help make affordable, ethical cornea transplants more readily available in the country.
Seeking to restore sight with 3D printing
The transplant that has taken place in Hyderabad represents a significant step forward for an approach to 3D printing corneas that has now been the subject of intense research for some time. Having been under R&D for at least seven years, Precise Bio’s 3D printed corneas gained fresh backing from Carl Zeiss Meditec last month, which could help ramp up the process of bringing them to market.
Going further back, Bangalore-based Pandorum Technologies 3D bioprinted cornea tissue in 2019, that was designed to enable the scarless healing of wounds in the eye. At the time, the firm’s disc-shaped implant was viewed as a potential way of treating those suffering vision loss due to corneal disorders, without having to rely on human donors.
In more practical applications, surgeons at Israel’s Shaare Zedek Medical Center (SZMC) successfully carried out the world’s thinnest artificial cornea transplant earlier this year. Developed by EyeYon, the 50 micron-thick implant was used to restore a local patient’s sight in a way that drastically reduced the time he had to wait for the procedure.
.
You might also like:
BellaSeno 3D printed breast implants enter human trials in Australia: BellaSeno’s 3D printed breast scaffolds are fully-resorbable, in that they’re designed to be implanted during breast regeneration, augmentation or revision surgeries, before being slowly absorbed by the body. In an initial trial last year, the firm proved the implant’s efficacy in human applications, hence it has now rolled-out two more trials that’ll see it used to treat chest or breast deformity patients directly.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment