Advanced Tissue Mimetic Technologies company Ronawk has partnered with B9Creations to develop and mass-produce 3D bioprinted hydrogels leveraging Ronawk’s Bio-Blocks.
Unlike traditional methods that force cells into artificial environments, Ronawk’s Bio-Blocks recreate conditions that allow cells to form tissues naturally. This results in tissue that closely resembles the native architecture and function of human and animal tissues.
As per Ronawk, Bio-Block cells maintain high levels of metabolism, viability, and proliferation, leading to superior production of biologics essential for advancements in biological therapies, diagnostics, prognostics, and countermeasures to biological threats.
“As a biotechnology company with a mission to accelerate next generation therapies, we needed to partner with a company that is as innovative and agile as we are in order to achieve our potential. B9Creations’ solutions business model and customizable hardware and software toolset enabled our team to move this effort from R&D into production and commercialization which will democratize access to healthcare research worldwide,” said Tom Jantsch, Ronawk President and COO.
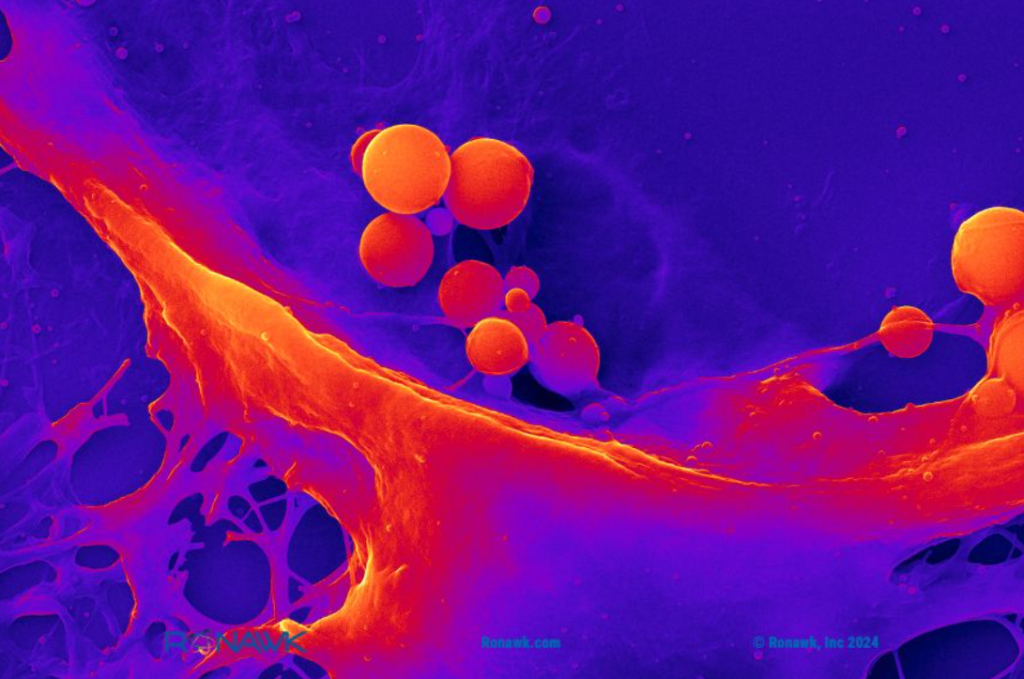 Bio-Blocks allow researchers to observe how a tissue-mimetic environment impacts processes like cell proliferation, extracellular vesicle (EV), and protein production. Image via B9Creations.
Bio-Blocks allow researchers to observe how a tissue-mimetic environment impacts processes like cell proliferation, extracellular vesicle (EV), and protein production. Image via B9Creations.
Mimicking natural tissues for accurate research
For decades, scientists have relied on plasticware to grow cells. However, this method has limitations. Plasticware is much stiffer than natural tissues and only allows cells to grow in a flat, two-dimensional layer, unlike the body’s three-dimensional environment. Gel-based scaffolds introduced later offered some improvements by enabling 3D structures but faced challenges such as inconsistency, difficulty in scaling, and more.
Offering several advantages, Ronawk’s tissue mimetic substrate allows scientists to introduce various stimuli to the cells, mimicking real-world cases. Additionally, it facilitates co-culturing, where multiple cell types can be studied together, providing a more holistic view of cellular interactions.
Moreover, the substrate enables the isolation of cells for detailed analysis of cellular signaling pathways. Notably, the Bio-Blocks’ form factor allows for continuous imaging throughout the experiment, providing invaluable real-time data on cellular behavior.
Ronawk combines scaffold-based and scaffold-free methods into an innovative approach. This enables initial cell growth with scaffold support, fostering the development of tissue-like structures that mimic organ formations. These structures promote healthier cell growth, authentic cell signaling, and natural tissue microenvironments.
According to Ronawk, its platform offers precise and consistent data on cellular responses, enhancing researchers’ ability to explore stem cell behavior. This capability accelerates drug discovery and the advancement of personalized therapies.
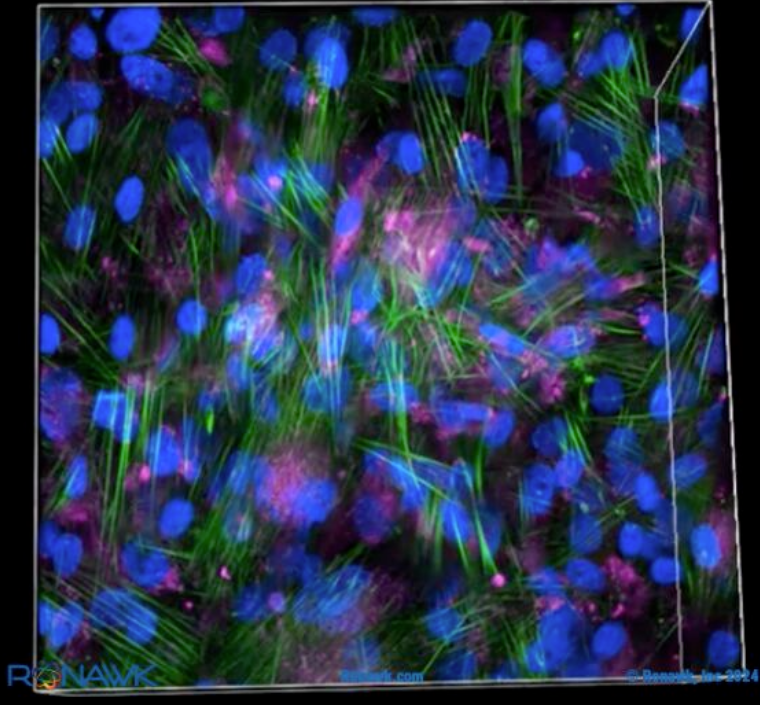
Applications of 3D printed hydrogels
Manufacturing on Demand
Hydrogels are essentially water-filled networks designed to resemble the extracellular matrix (ECM) found in the human body. The ECM provides structural support and biochemical cues for cells, making hydrogels ideal for creating scaffolds that encourage tissue growth. Previously, scientists and institutes have conducted research regarding hydrogel and its applications.
One notable example includes Canada’s Laval University researchers developing a novel 3D printed hydrogel for treating cervical cancer. Consisting of a thermosensitive polymer and gold nanoparticles, the gel was designed to be applied locally to carry drugs to the cervix.
The team aimed to use scan data to customize the hydrogel to individual patients’ anatomies, enhancing treatment and recovery. Initial tests showed promising results in drug delivery and cytotoxicity. The researchers planned further in-vivo studies to track implant degradation and improve cervical cancer therapies.
Researchers from University of California Los Angeles’ (UCLA) Samueli School of Engineering developed a two-part process process to improve hydrogel strength for potential use in artificial tendons, ligaments, and cartilage. The synthetic biomaterials replicate natural tissue structure, stretchiness, and durability, and their flexibility enables new 3D printing configurations.
Using freeze-casting and salting-out treatments, the hydrogels achieved ten times the toughness of natural tissues. These hydrogels, tested over 30,000 stretch cycles without deterioration, were deemed suitable for biomedical applications and advanced bioelectronics.
Elsewhere, researchers from the University of Illinois developed a functional walking “spinobot” by merging a 3D printed hydrogel skeleton with a rat’s spinal cord. The team used glutamate, a neurotransmitter, to stimulate muscle contractions in the spinal cord, which moved the robot’s “feet.”
According to the researchers, this method not only facilitated movement but also mimicked aspects of the peripheral nervous system’s development. It opens possibilities for future designs integrating spinal sensory inputs for control mechanisms in soft robotics.
You might also like:
Ricoh’s new medical 3D printing facility to save time in the operating theater: Based at Atrium Health Wake Forest Baptist, a medical center in North Carolina, the facility provides clinicians direct access to development, design and manufacturing capabilities for 3D printing FDA-approved anatomic models. The patient-specific devices can be used for surgical planning and patient education.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Ada Shaikhnag

Leave A Comment