The new drug delivery system developed by global engineering company Renishaw has been successfully used to expand part of clinical trials for the treatment of Parkinson’s disease in humans. Renishaw designed and manufactured a chronic drug delivery system called nerve infusion, which consists of up to four catheters, which can be accessed through a 3D printed titanium port implanted behind the patient’s ear to help pass through The brain infusion delivers the new medicine safely to the patient. Neurodegenerative diseases.
It was originally created to enable drugs to bypass the blood-brain barrier for the continuous treatment of neurological diseases and brain tumors. Nerve injection is a mechanism that delivers drugs directly to the brain, so new treatment methods can be developed to treat Nervous system diseases Renishaw claims that the smallest dose provides the greatest effect.
It is expected to obtain an innovative patented design that enables patients to receive infusions in an outpatient setting instead of requiring a new catheter to be implanted for each infusion. This is the only option for many patients so far. According to experts, infusion therapy is usually more effective than long-acting pills and patches in the treatment of Parkinson’s disease, especially when neurodegenerative diseases continue to develop, and compare with the benefits of the drug which make the patient suffer from the so-called “regression” symptoms before the next dose, infusion therapy is particularly effective. Instead, infusion therapy provides a continuous flow of medication throughout the day, addresses the lack of dopamine in the brain, and treats patients with motor and non-motor symptoms.
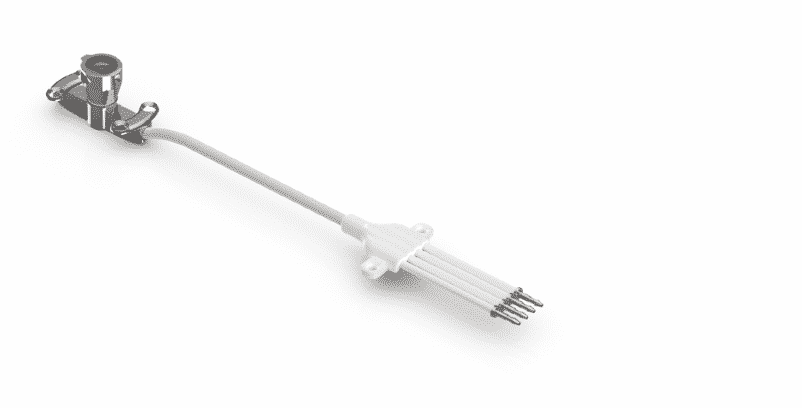
The neuroinfuse chronic drug delivery system consists of a maximum of four catheters, which are implanted into the target area of the brain. The catheter can be accessed through a 3D printed titanium percutaneous bone anchor port implanted behind the patient’s ear. The port then connects the internal catheter to the external dosing kit and is designed to allow intermittent chronic re-dosing of the therapeutic agent without the need for further surgery. Use an MRI-compatible set of applications to connect the infusion line filled with medication, which can be located on the port repeatedly. When the retractable needle extends through the septum in the port, the therapeutic agent in the external infusion line can be injected through the implanted catheter.
The port is made on Renishaw’s own metal 3D printing system and has a rough surface under the skin to promote bone integration and fixation of the device, while above the skin it is polished to prevent bacterial sedimentation. After the implantation operation, immediately press the silicone cap and sterile dressing on the port to prevent thickening of the skin around the port and facilitate the connection of the fixation device.
“This device has the potential to completely change how neurological diseases are treated. The port allows repeated delivery of pharmaceuticals over long periods of time, without the need for further surgery — patients can be admitted as outpatients for the infusions,” described Max Woolley, Technical Fellow and Head of Drug Delivery Device Research and Development at Renishaw in 2019. “If we produced the device using traditional subtractive machining, we estimate it would have required up to ten parts. By designing for metal additive manufacturing, it was possible to produce it as one component, reducing the time needed for manufacture and the potential for error from unnecessary assembly operations.”
The company recently announced that its intraparenchymal drug delivery system played a key role in a phase 1/2 clinical trial joint study conducted with Finnish pharmaceutical company Herantis Pharma, which recruited 17 people with moderate Parkinson’s disease Everyone has 15 years of evidence of motor symptoms. The study explored the safety, performance, and tolerability of Renishaw’s nerve injection device, as well as a research therapy called brain dopamine neurotrophic factor (CDNF), which is based on the blood and fluid that naturally exists around the brain and spinal cord The protein in Parkinson’s disease. And the surgical accuracy of the device implantation procedure.
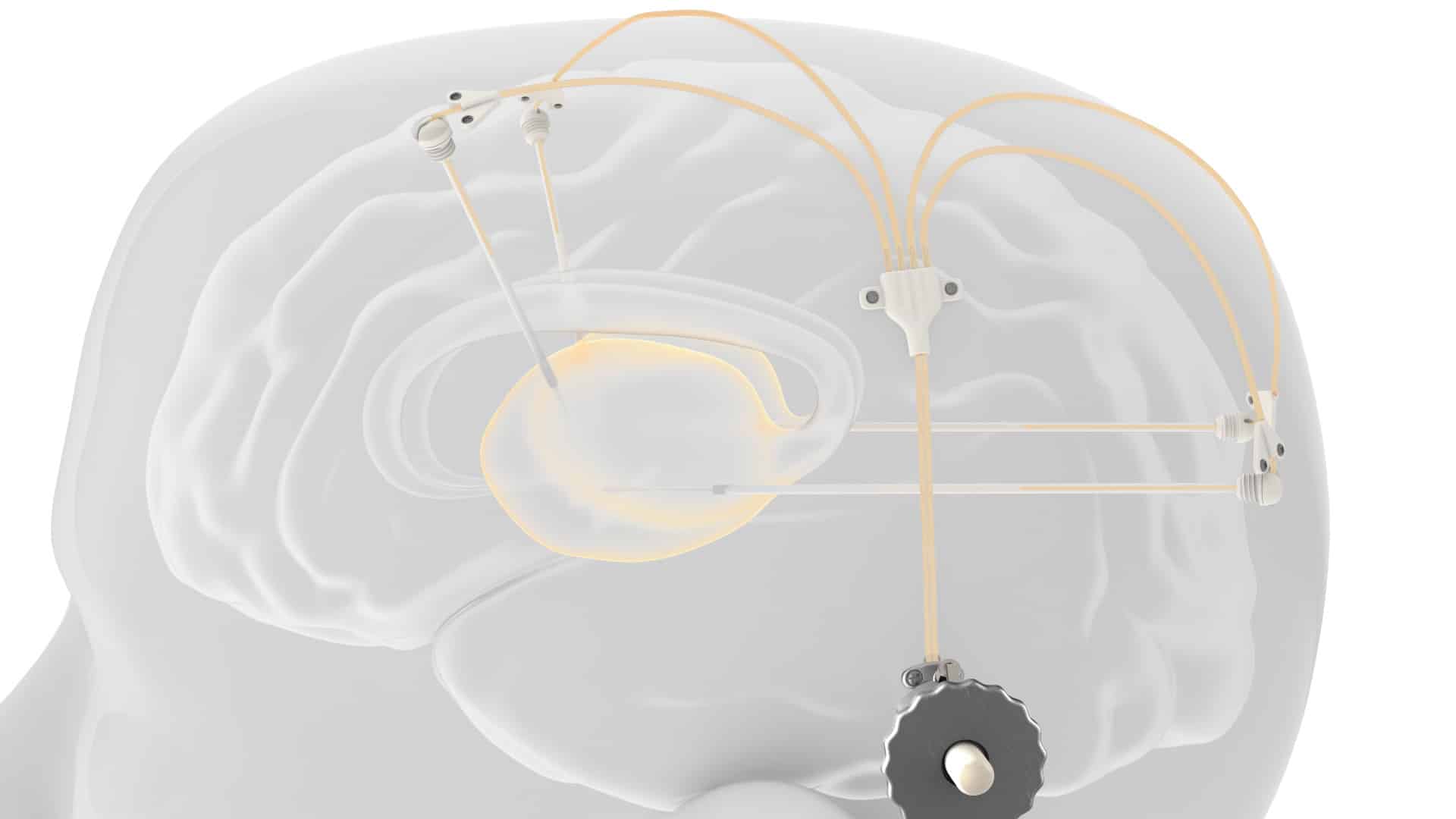
During the trial, CDNF was delivered directly to the brain of the patient through a delivery system provided by Renishaw Neuro Solutions. At present, only drug delivery devices that can only be implanted by neurosurgeons are considered to be the only platform that allows repeated and intermittent infusion into the functional tissues of the brain, called parenchyma. The preliminary results of the main research show that the device can be placed predictably and accurately, and has positive performance and safety.
In addition, as part of the extended study, nerve infusion continues to be safely and effectively infused into the brain, proving that patients with the moderate disease continue to be well tolerated the treatment of Parkinson’s disease, showing the potential to relieve motor symptoms and slow disease progress, as reported by Herantis Pharma. In fact, Renishaw claimed that the results of the 15 patients who entered the extended study showed that the device was safe and there were no serious adverse events believed to be causal to the device or the drug. In addition, the unique repeat infusion capability of the system continues to facilitate the evaluation of the safety and early efficacy of CDNF in patients.

“I’m delighted to see Renishaw’s drug delivery system continuing to facilitate repeated infusions over an extended period of time for such a complex condition as Parkinson’s Disease,” said Rupert Jones, Managing Director of Renishaw Neuro Solutions. “The device’s performance demonstrates what a powerful delivery platform it is for the treatment of many, currently incurable, neurological conditions, opening new possibilities in the field of neurosurgery and neuroscience. I see this as a hugely positive step forward and believe all involved in the study should be proud of their achievements.”
As a novel infusion protocol, nerve infusion is currently only used in an approved clinical trial setting. However, in order to finally make the device universally available to patients, Renishaw is seeking academic, clinical, and industrial partners in various indications from oncology to neurodegenerative diseases. However, the company hopes that the drug delivery system has potential applications in a series of comprehensive therapies such as gene therapy, gene therapy, cell therapy, drug therapy, protein therapy, and enzyme therapy, especially for enhancing neurodegeneration, neurotumor, and other Therapeutic conditions for neurological diseases that make neurasthenia. More importantly, in the short term, Renishaw is seeking to open a path from laboratory to clinical for effective and cost-effective Neurotherapy.


