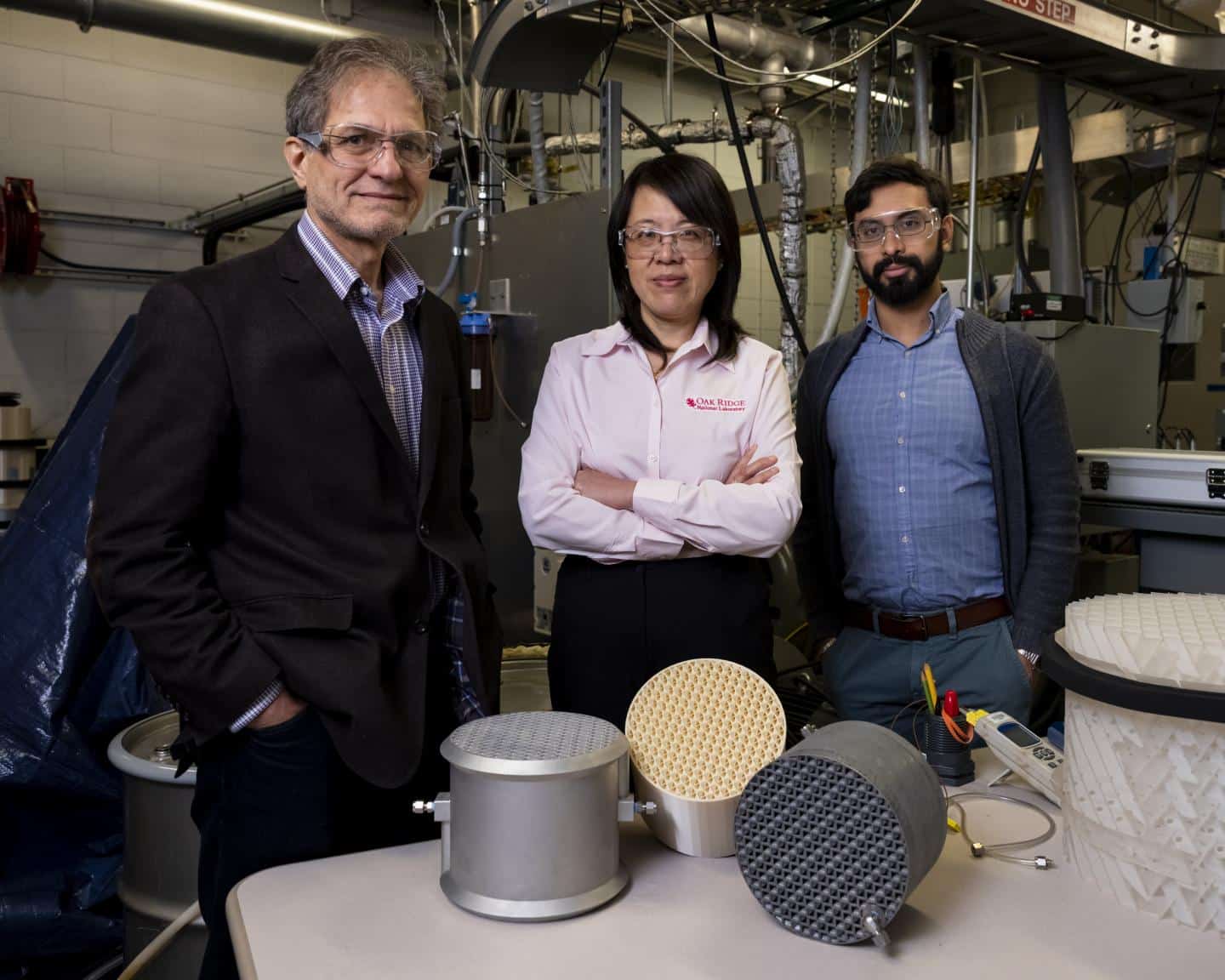
A team from Oak Ridge National Laboratory invented a 3D printed absorption device made of aluminum that can capture carbon dioxide emissions from fossil fuel plants. This innovative device can also be used in other industrial processes to help curb global emissions of greenhouse gases such as CO2, which trap heat into the atmosphere and contribute to global warming.
The 3D printing equipment consists of a heat exchanger with a mass exchange contactor and integrates internal coolant channels to improve heat exchange. The final prototype size of the device is 20.3 cm in diameter, 14.6 cm in height, and total liquid capacity of 0.6 liters. Because of the material’s high thermal conductivity, structural strength, and printability, it is printed with aluminum, although other materials can also be used.
The height of the CO2 capture device was tested inside the absorption tower. The height of the absorption tower was 1 meter and the width was 8 inches. The 3D printed enhancement is placed in the upper half of the column between some of the seven filling elements of the structure. 3D printing is essential for creating parts that can fit into the chromatographic column, conforming to the geometry of the packing elements, and optimizing the contact surface area between air and liquid streams.
Costas Tsouris, one of the main researchers of the ORNL project, explained: “We call this device an enhanced device because it can enhance mass transfer (the amount of CO2 transferred from gas to liquid) through in-situ cooling.” “Controlling absorption Temperature is essential for capturing carbon dioxide.”
In the research project, the ORNL team set out to solve the specific problems of the traditional CO2 absorption process using solvents. Generally, when a gas reacts with a solvent, these processes generate heat, which can inhibit the efficiency of the entire process. By using 3D printing, researchers can design a customized device that can use coolant channels to radically reduce the temperature rise caused by the solvent reaction in the column.
“Before designing the 3D printing equipment, it was difficult to implement the heat exchanger concept in the CO 2 absorption tower due to the complex filling geometry of the CO2 absorption tower,” said Xin Sun, the main researcher of the project. “Through 3D printing, mass exchangers and heat exchangers can coexist in a multifunctional enhanced device.”
Lonnie Love, ORNL’s principal manufacturing researcher who designed the device, added: “The device can also be manufactured using other materials, such as emerging high thermal conductivity polymers and metals. Over time, additive manufacturing methods such as 3D printing It is usually cost-effective because it requires less effort and effort to print parts compared to traditional manufacturing methods.”

In tests, the 3D printing device performed well inside the chromatographic column: it enhanced the system’s ability to use amine solutions to capture carbon dioxide. The research team actually conducted two types of tests to find out which operating conditions would give the best results: one was to change the flow of CO2 containing gas, and the other was to change the flow of solvent. Finally, compared with conventional absorption, both methods have been improved, but the results are related to the gas flow rate.
“The success of this 3D printed intensified device represents an unprecedented opportunity in further enhancing carbon dioxide absorption efficiency and demonstrates proof of concept,” Sun concluded. Going forward, the ORNL team will seek to optimize the operating conditions and the device’s geometry for even better carbon absorption. The groundbreaking research was recently published in the AIChE Journal.

