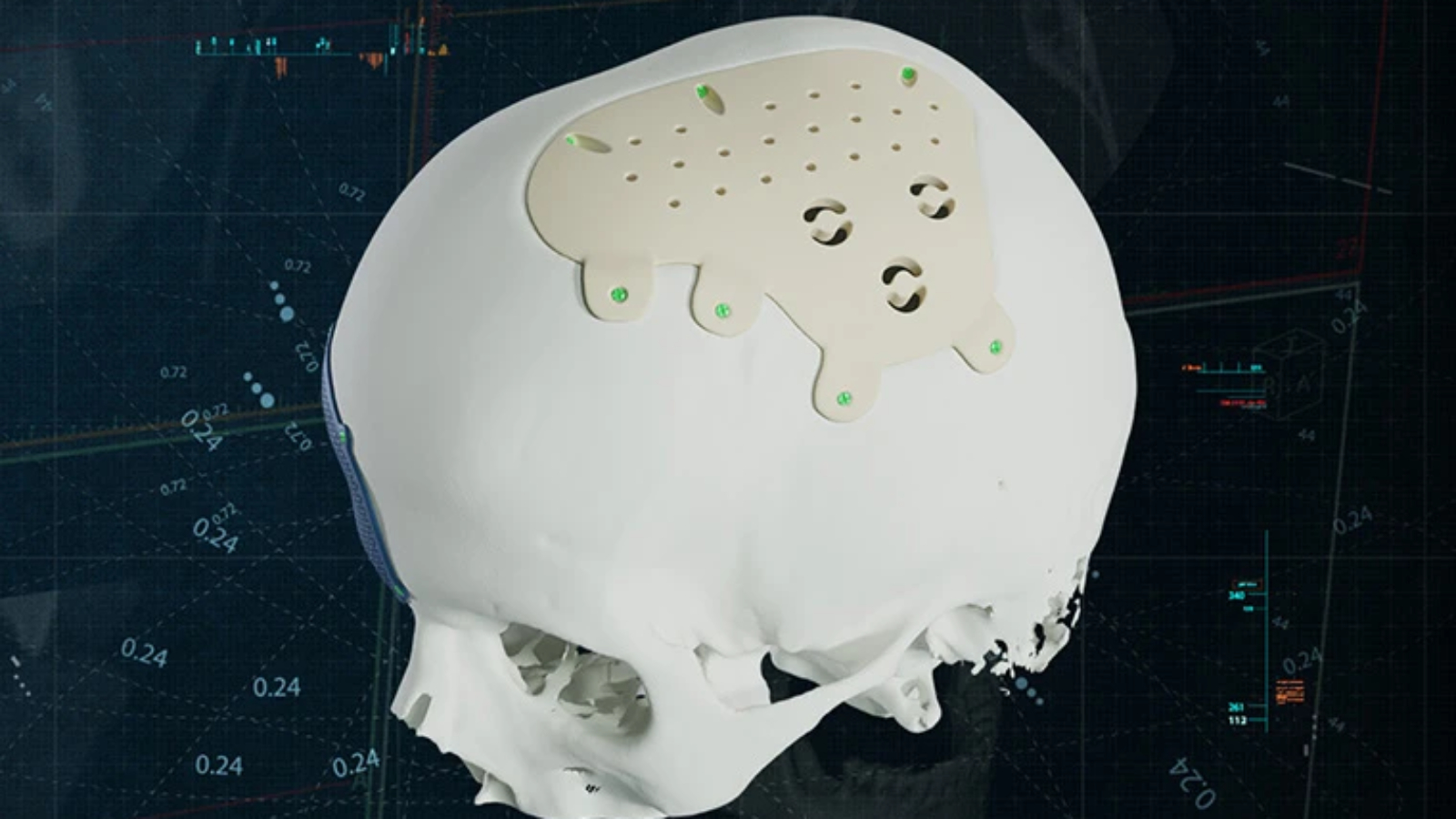
Materialise NV, a Belgium-based 3D printing software and medical device company, has introduced custom-made implants made from polyetheretherketone (PEEK) to its cranio-maxillofacial (CMF) portfolio. Surgeons can now select either titanium or the high-performance polymer for patient-specific reconstructions while using the same digital planning and case management system. Availability covers Europe, excluding Switzerland.
Integration occurs within the company’s established CMF platform. Surgical planning is conducted collaboratively in Mimics Enlight CMF software with dedicated clinical engineers. Case management runs through Mimics Flow, and fixation is supported using Materialise Standard+ Solutions. Validation and traceability procedures remain unchanged regardless of material selection.
“Personalization isn’t just about material choices but about the precision of the planning process and the reliability of execution,” said Maarten Zandbergen, Market Manager at Materialise. “Our clinical engineers work hand in hand with surgeons to design each implant, and that collaborative approach remains constant whether the case calls for titanium or PEEK. What changes is the surgeon’s freedom to choose; what stays the same is the peace of mind that comes from a proven, end-to-end process.”

The polymer option expands an existing titanium 3D printed implant portfolio. Radiolucent properties reduce imaging artifacts in postoperative scans, a characteristic often associated with cranial reconstruction, facial contour restoration, and onlay applications. Titanium remains widely used in load-bearing indications due to mechanical strength and long-standing clinical use. Both materials are offered through the same planning and approval process.
Production of the new implants takes place in Europe through Ad Mirabiles, Materialise’s manufacturing partner. Ad Mirabiles operates under an EN ISO 13485 certified quality management system for medical devices. Materialise states that implants made from the polymer are delivered within 72 hours following surgical plan approval, aligning with turnaround times established for its titanium offering.
Headquartered in Belgium, Materialise has more than three decades of experience in 3D printing software and services. Activities include medical device production and software development serving healthcare, automotive, aerospace, eyewear, art and design, wearables, and consumer goods sectors.
Manufacturing on Demand

Regulatory and production constraints shape implant material adoption
Medical implant manufacturing operates under strict regulatory and quality system requirements. In March 2025, a patient-specific facial implant was manufactured from polyetheretherketone at the University Hospital Basel using 3D Systems’ EXT 220 MED extrusion platform. The device was described as the first MDR-compliant 3D printed facial implant produced at the point of care. Fabrication took place within a hospital cleanroom environment using VESTAKEEP i4 3DF material from Evonik. Deployment of such systems requires validated workflows, controlled processing environments, and adherence to European medical device regulations. Since its launch in August 2023, the EXT 220 MED platform has been used in more than 80 cranial implant surgeries, illustrating the procedural and certification requirements necessary to shift production closer to surgical settings.
Process control requirements similarly constrain titanium implant systems used in orthopedic reconstruction. restor3d’s Ossera AFX ankle fusion cage platform combines standardized and custom configurations manufactured in 3D printed titanium alloy with porous architectures intended to support bone integration. Production of complex porous geometries demands precise parameter control and consistent mechanical performance under regulated quality systems. Operational considerations extend beyond fabrication, including sterile packaging, inventory management across standardized and made-to-order implants, and integration into established surgical workflows. These factors influence how new materials and geometries are incorporated into clinical practice, particularly when validation, traceability, and manufacturing repeatability must remain consistent across cases.

You might also like:
MIT Introduces MagMix to Reduce Cell Settling in 3D Bioprinting, Addresses Key Limitation: The project received support from MIT’s Safety, Health, and Environmental Discovery Lab (SHED), which provides technical infrastructure and interdisciplinary expertise for scaling lab innovations. “MagMix is a strong example of how the right combination of technical infrastructure and interdisciplinary support can move biofabrication technologies toward scalable, real-world impact,” says SHED founding director Tolga Durak.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Anyer Tenorio Lara

Leave A Comment