Welsh biotech firms Jellagen and Copner Biotech have deployed a novel 3D bioprinting process to turn bio-inks made from jellyfish collagen into “world-first” functional soft tissue structures.
Using Jellagen’s Collagen Type 0 bio-ink and Copner Biotech’s GRAPE S1 3D bioprinter, the pair have come up with a process of creating functional tissues from man-made materials and living cells. While many existing bioprinting methods rely on mammalian cells, which can come with ethical considerations, the firms say theirs could offer a less contentious way of engaging in tissue engineering R&D.
“We are delighted with the data outputs of this project that have clearly demonstrated the further potential use of our Collagen Type 0 biomaterials for future bio-ink development,” said Jellagen Founder, Professor Andrew Mearns Spragg. “We are very excited to see how this technology can be further developed towards medical tissue engineering applications and cell culture research market solutions.”
Copner Biotech’s GRAPE S1 system
Established in 2020, Copner Biotech specializes in the R&D of 3D cell culturing and other related technologies. Initially, all the Welsh start-up had was a concept for a ‘next-generation 3D printing technology,’ potentially capable of creating PETG scaffolds. However, these scaffolds, which require low numbers of cells for seeding, have since exhibited the ability to optimize cell capture and attachment.
This development saw the company presented with the Global Health and Pharma International Life Sciences Award for Innovation in July 2021. Copner Biotech is also said to have entered into “several high-value research projects,” with Welsh Government support, including most recently, its 3D bioprinting work with Jellagen.
As announced in October 2021, the aim of Jellagen and Copner Biotech’s partnership is to optimize the performance of the GRAPE S1 extrusion 3D bioprinter. The system works by using microfluidic droplet deposition to create layers via sequential printhead movement, in a process that’s designed to yield a high level of print precision and control.
Rather than relying on traditional STL or G-code files, Copner Biotech’s next generation bioprinter uses graphical rectangular actual positional encoding or ‘GRAPE’ files. These are programmed to tackle troublesome data approximations, modeling anomalies and low accuracy levels, in a way that enables the production of repeatable cell-based structures.
Another benefit of the GRAPE S1 is that it prints via material collected from an open-source microfluidic reservoir, allowing users to utilize cell combinations of their choice. Copner Biotech anticipates that the system’s open-material setup will soon “empower the research community to push the boundaries of 3D bioprinting,” and working with Jellagen, it has now begun realizing the technology’s potential.
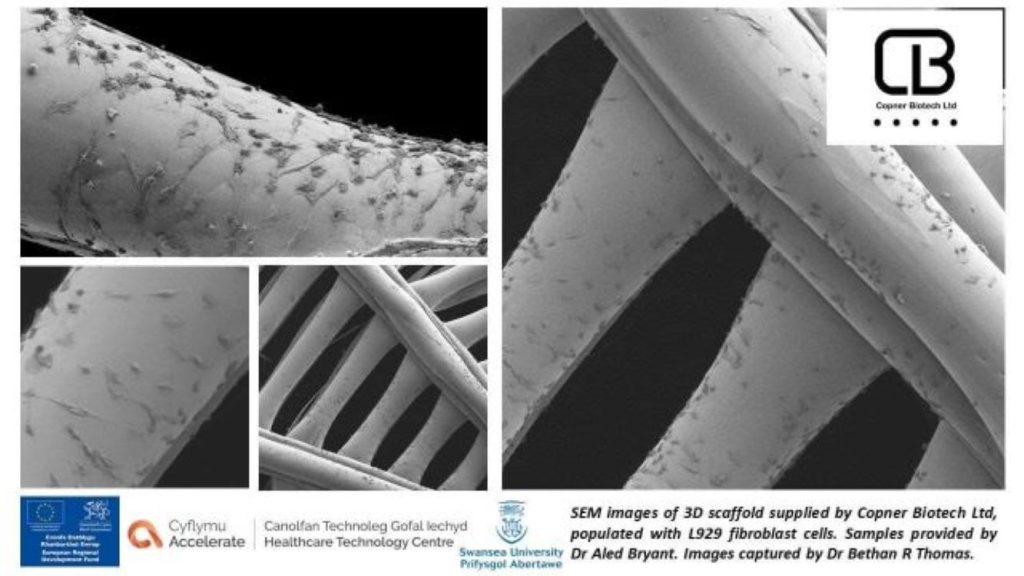
A Welsh 3D bioprinting R&D initiative
Manufacturing on Demand
According to Jellagen and Copner Biotech, many current bioprinting systems on the market are limited by “dated software protocols,” and rely on mammalian sources of biomimetic materials and living cells. Backed by Wales’ business R&D investor scheme, SMARTCymru, the firms’ project has seen them collaborate since last year, on developing an alternative from Jellagen’s next-generation biomaterial.
As this Collagen Type 0 bio-ink is harvested from Barrel Jellyfish in the Irish Sea, which are fast becoming pests off the coast of the UK, the firm has positioned its production as a way of maintaining biodiversity. In theory, as the material is sourced from the root of the evolutionary tree, Jellagen says Collagen Type 0 is safer, purer and more effective and versatile than mammalian counterparts.
Utilizing Collagen Type 0 as a bio-ink, the companies say they’ve now managed to 3D print very fine, less than 100m diameter tissue structures, on Copner Biotech’s GRAPE S1. In combination with an advanced algorithm, this process is also said to allow for the precise modeling of 3D architectures, to the point they could be made to mimic the organic micro-environments of a living system.
Moving forwards, the project participants believe their workflow could be of particular importance in bioscience R&D, within the creation of repeatable 3D cell culture models. Though not designed to 3D bioprint transplantable structures, Copner Biotech CEO Jordan Copner says the technology it has developed with Jellagen, has the potential to “transform lives through tissue engineering.”

Bio-inks in the field of 3D bioprinting
Cell-depositing hardware isn’t the only area of 3D bioprinting that continues to be the subject of intense research, and scientists have made a number of bio-ink R&D advances in recent years. Researchers at the Irish RCSI University of Medicine and Health Sciences have come up with a wound-healing 3D bioprinting ink that can be turned into regenerative tissue scaffolds, which mend skin without scarring.
This followed the development of a new silk-based 3D printing bio-ink at Japan’s Osaka University, which was said to enable the fabrication of cell-laden structures with improved printability. During testing, the scientists’ material was found to minimize the internal stresses placed on cells during 3D printing, which improved their survivability and allowed them to retain complex bio-mimicking shapes.
More recently, a team at Cornell University have managed to develop a 3D printable biohybrid composite that can be used to create artificial skin, which replicates the behavior of its real-life counterpart. Soft and biocompatible, but also flexible enough to withstand continued distortion, it’s thought the material could one day be used to fabricate scaffolds from patients’ cells that heal wounds in-situ.
You might also like:
SprintRay to expand into new territories after raising more than $100M: Dental 3D printer manufacturer SprintRay has raised over $100 million in Series D funding, according to a Deal Street Asia report.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment