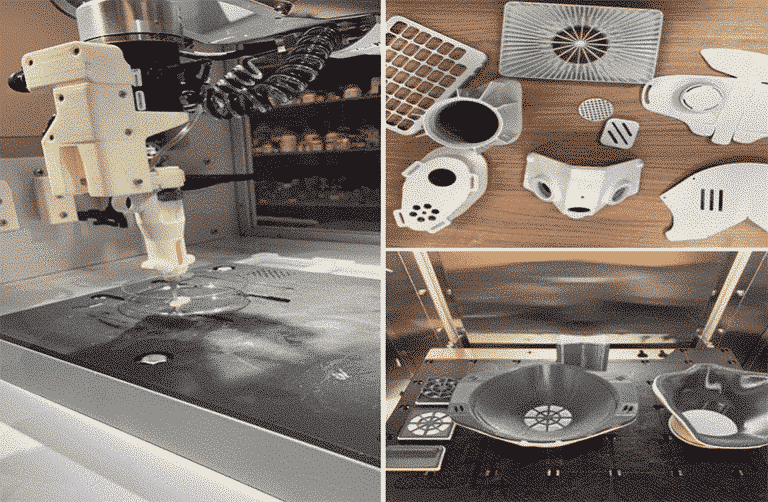
Intrepid 3D printing firms and those who wish to print masks, gowns and other personal protective equipment (PPE) to combat the coronavirus pandemic, may want to look at the FDA’s new frequently asked questions webpage on this issue.
Stating that it welcomes attempts to fight coronavirus, the bureau stated that the 3D printed masks will probably not offer air filtration security as those FDA-cleared surgical masks and N95 respirators, but the latter ones are in short supply.
Many colleges and research groups have been rushing to create DIY ventilators to assist patients who cannot get one.
FDA advised people using the same specifications, dimensions, and performance as the original parts.
The bureau also encouraged people to work together with MedTech manufacturers for advice.
Atlanta-based Global Center for Medical Innovation (GCMI) announced this week it is entering into a cooperation with numerous entities to supply designs — with no charge and also necessary regulatory advice — for any Good Manufacturing Practice (GMP) – compliant facility to utilize in the creation and supply of face guards to healthcare employees.
The FDA recently issued an emergency use authorization (EUA) for ventilators, ventilator tubing connectors, and ventilator accessories, which might include items like 3D printed tube connectors for multiplexing ventilator usage.
The bureau is also cooperating with the Department of Veterans Affairs (VA) Innovation Ecosystem, America Makes public-private partnership, along with the National Institutes of Health (NIH) 3D Print Exchange, a resource in the National Institute of Allergy and Infectious Diseases at the NIH.
The FAQ on apparatus for the coronavirus of the FDA is available here.