Medical technology company Cairn Surgical has secured ISO 13485:2016 certification for its Quality Management System for the Breast Cancer Locator (BCL) System, enabling the company to advance scaled production and accelerate broader commercialization efforts.
“ISO certification is an important step in the evolution of the company as we look towards broader commercialization of the BCL System in Europe and entrance into new regions,” said Cairn Surgical CEO David Danielsen. “It demonstrates not only our commitment to product quality, but also our ability to achieve it consistently across the design, development and manufacturing of our BCL System, and compliance with all applicable regulatory requirements. We look forward to the momentum this milestone will bring to our regulatory and market access efforts.”

Advanced Tumor Mapping for Improved Breast-Conserving Surgery
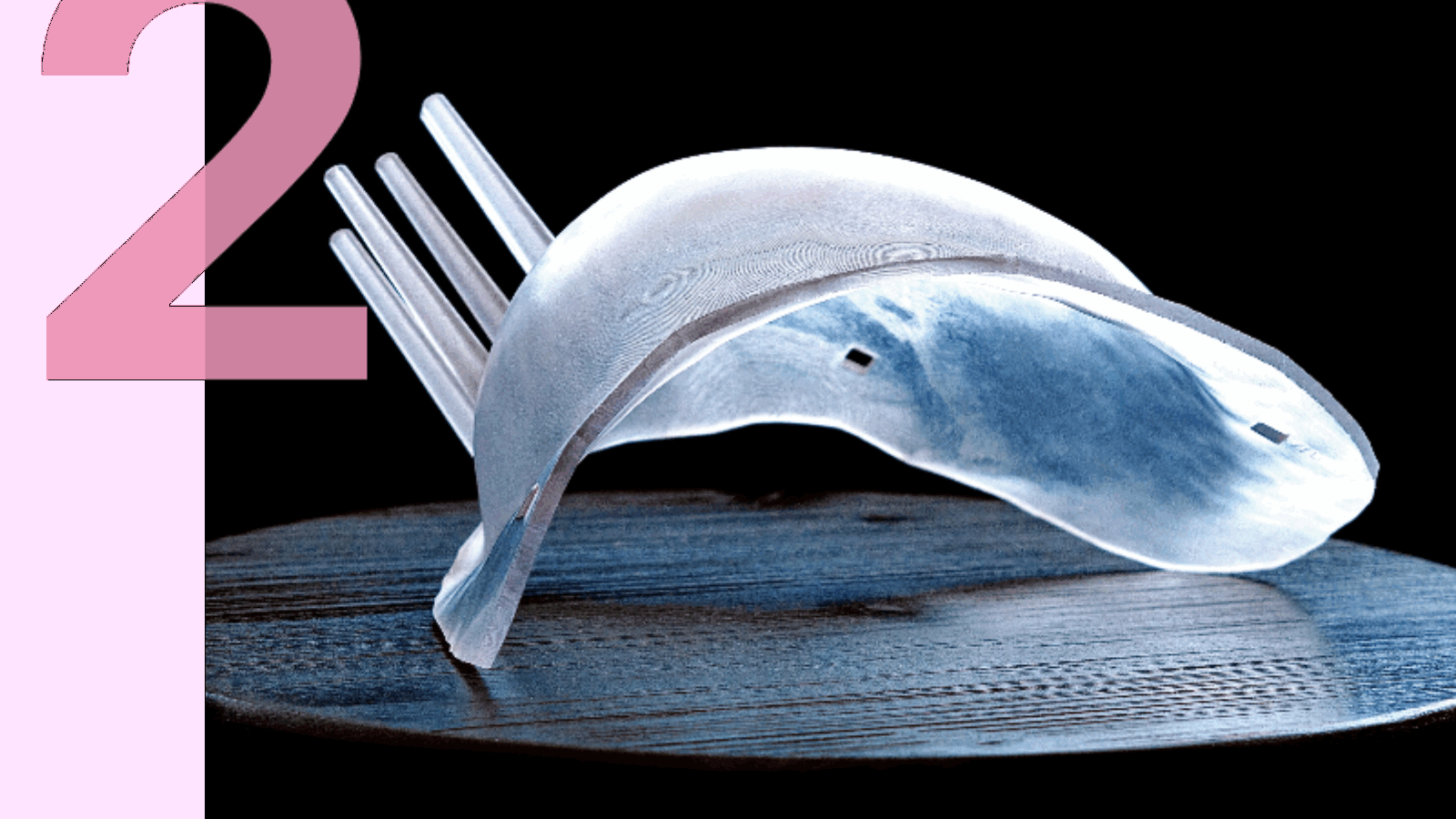
The BCL System is designed to improve the precision of breast-conserving surgery by providing surgeons with detailed, patient-specific guidance on tumor shape, size, and location—critical information often lacking in traditional wire localization methods. The goal is to help surgeons achieve clear margins while preserving healthy tissue.
The system integrates two components generated from a supine MRI taken with the breast in surgical position. A customized, 3D printed form—the BCL—is created to fit the patient’s anatomy and outline the tumor’s boundaries. Simultaneously, the Visualizer provides a real-time, interactive 3D image of the tumor within the breast. Used together during surgery, these tools offer surgeons precise reference points to guide excision.

Cairn Surgical announced it is currently conducting a clinical trial of the BCL System in the U.S., with results expected later this year. The findings are expected to support further regulatory submissions and pave the way for market expansion.
Recent ISO Certifications in MedTech
Manufacturing on Demand
Other companies across the medical technology landscape are also advancing with ISO certification to strengthen regulatory compliance and market positioning.
Lithoz, a ceramic 3D printing company headquartered in Vienna, has secured ISO 13485 certification for its quality management system, aligning its operations with international standards for medical device manufacturing. The certification, regarded as the benchmark for regulatory compliance in healthcare production, enables Lithoz to meet FDA Quality System Regulation (QSR) requirements and expands its role in supplying 3D printed components for medical and dental applications.
In 2019, BellaSeno, a German medical startup, obtained ISO 13485 certification for its 3D printed breast implants, branded as Senealla. That same year, the company secured an additional €1 million in funding from existing investors, bringing total investment to €4.2 million. CEO Simon Champ noted that the certification and funding would support upcoming clinical trials and broader commercialization: “We are offering the entire process under ISO 13485 certification to other medtech companies worldwide, from concept and in-house design to manufacturing of prototypes, clinical trials and series production.”
You might also like:
KAIST’s breakthrough brain model tracks neural activity for 27 days: Researchers at Korea Advanced Institute of Science and Technology (KAIST) have 3D printed brain-like tissue with a multilayered structure, alongside an integrated system to monitor neural activity in real time.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paloma Duran

Leave A Comment