German medical device manufacturer BellaSeno has announced that its proprietary 3D printed breast implants have entered human trials.
BellaSeno’s 3D printed breast scaffolds are fully-resorbable, in that they’re designed to be implanted during breast regeneration, augmentation or revision surgeries, before being slowly absorbed by the body. In an initial trial last year, the firm proved the implant’s efficacy in human applications, hence it has now rolled-out two more trials that’ll see it used to treat chest or breast deformity patients directly.
“This trial is a very important step for us to demonstrate that our approach is not only safe, but leads to substantial improvements in terms of long-term safety, health and quality of life,” said Dr. Tobias Grossner, CMO of BellaSeno. “We very much hope the trials once more confirm our scaffold-guided tissue reconstruction concept.”
 BellaSeno’s 3D printed bioresorbable Senella breast scaffold. Photo via BellaSeno.
BellaSeno’s 3D printed bioresorbable Senella breast scaffold. Photo via BellaSeno.
BellaSeno’s resorbable implants
Working from its bases in Leipzig, Germany and Brisbane, Australia, BellaSeno has come up with a unique biomimetic design platform that enables the 3D printing of clinically-validated resorbable polymers into implants. Designed to serve as an alternative to the complex surgical methods and stem cell-based science behind skin grafts, the firm’s portfolio revolves around its Senella Breast implant.
So far, in preclinical studies, the scaffold has proven capable of resorbing around 2-3 years after surgery, and allowing for fat injection from any angle, making it easier for surgeons to use. Compared to the silicone prostheses deployed in more than 70% of breast surgeries, the firm’s implant is also demonstrably over 90% lighter, while its ability to achieve full tissue integration means less risk of complications.
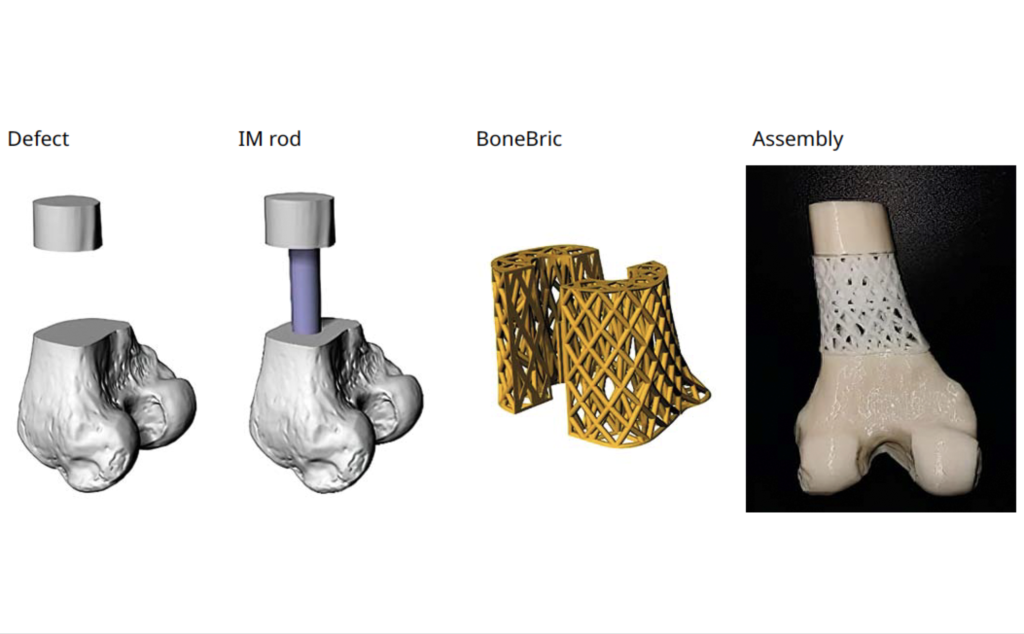
Other products in BellaSeno’s portfolio include the Senella Pectus, an implant designed for the treatment of chest wall defects, in a way that’s not as complex and less risky than traditional Nuss or Ravitch procedures. The company also markets its 3D printed BoneBric bone graft-holding cage, a device that effectively secures implants within bone voids and segmental defects during the healing process.
Over the last three years, these products have passed several milestones on their journey towards end-usage and commercialization. In July 2019, BellaSeno raised €4.2 million and announced that its implants had gained ISO 13485 certification. Shortly after, BellaSeno’s breast implants entered clinical trials using Evonik RESOMER material, and it has since progressed further into human evaluations.
Entering human trials down under
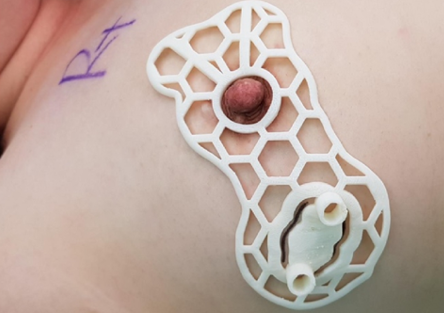
Already, recruitment is said to have started for the human trials of BellaSeno’s 3D printed implant in Brisbane, Australia. Sponsored by its Australian subsidiary, BellaSeno Pty, the research program is set to see the company’s breast scaffolds deployed in two different applications, the first of which will see it used to treat ‘pectus excavatum.’
As part of the study, ten sufferers of this congenital chest wall deformity, which causes the breastbone to sink into the chest, will have porous polycaprolactone scaffolds implanted into them. Once inserted in compressed form via minimally invasive surgery, the devices are expected to unfold and merge with patients’ own fat tissue obtained via liposuction, leaving no foreign material traces behind.
Manufacturing on Demand
In the second of these trials, BellaSeno aims to enrol another twenty patients that either require breast implant revision or congenital breast defect surgery, who will be treated with implants made from the same absorbable suture material.
Last year, the firm published its first-in-human data, which demonstrated that its implant was able to camouflage a pectus excavatum defect in a way that isn’t possible via conventional techniques. Through its latest trials, BellaSeno now aims to further assess its scaffold’s safety and adverse event rate, as well as the changes in fat volume, soft tissue retention and any pain it causes to patients.
“We are delighted to spearhead the administration of novel, fully-absorbable implants for soft tissue reconstruction applications,” said Dr. Michael Wagels, principal investigator of the pectus excavatum trial. “There is a clear need for a safe alternative to contemporary alloplastic materials and operative techniques in pectus excavatum and breast surgery.”
“Having customizable implants that resorb, leaving behind the patient’s own tissues, is a big step forward.”
BellaSeno is just one of several commercial and academic organizations seeking to use 3D printing as a means of optimizing breast surgeries. Scientists at Asan Medical Center have developed 3D printed breast surgery guides, designed to help cancer survivors retain as much breast tissue as possible post-tumor removal.
Elsewhere, in the field of 3D bioprinting, CollPlant and CELLINK are 3D bioprinting breast implants that degrade over time, in a way that allows them to be replaced by patients’ native tissues. While the tissues haven’t quite reached the human trial stage just yet, if they do, they’re expected to address a $2.8 billion market.
Similarly, Healshape raised $6.8 million towards 3D bioprinted breast tissues of its own earlier this year. Made from patients’ own cells, the implants are being developed to help treat those women who have undergone a mastectomy, by providing them with a patient-specific graft that’s less likely to be rejected.
.
You might also like:
Scientists say India’s first 3D printed cornea “can be used in humans” after successful animal trial: Made from a bio-ink derived from human donor tissues, the artificial material and animal residue-free cornea was transplanted into the eye of a rabbit. Due to its “completely natural” base, the implant’s developers say it could soon be “used in human beings,” to treat corneal scarring as well as other serious eye conditions that can cause blindness.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment