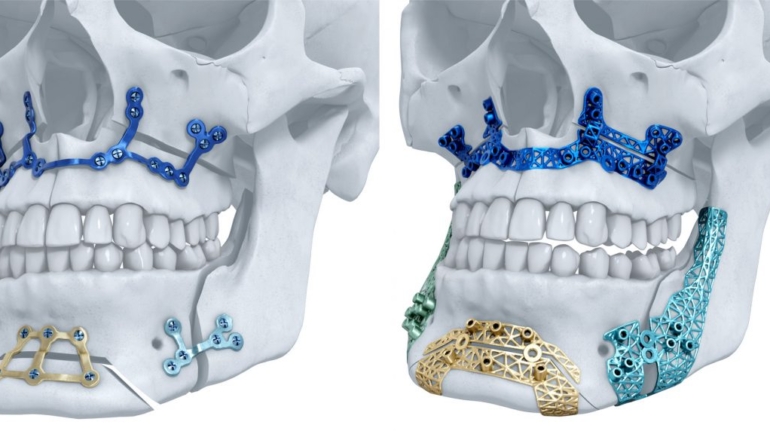
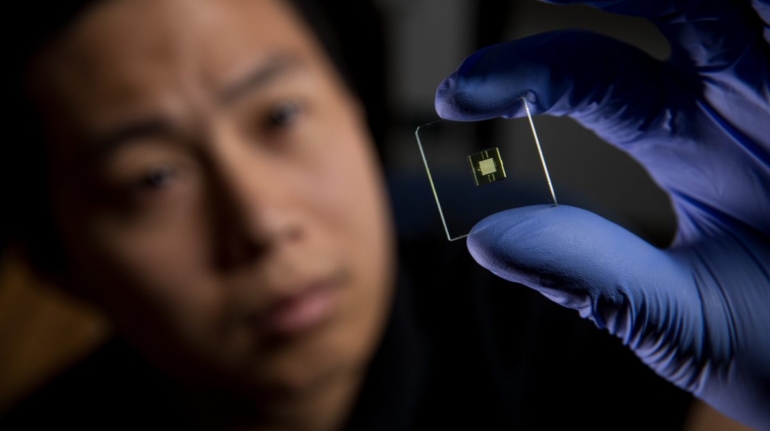
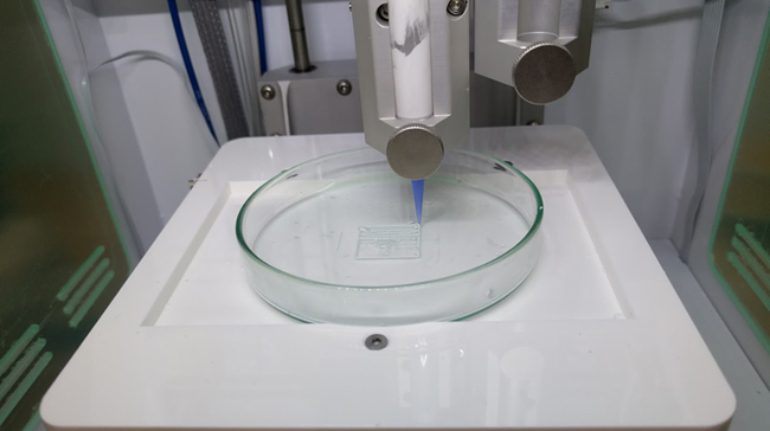
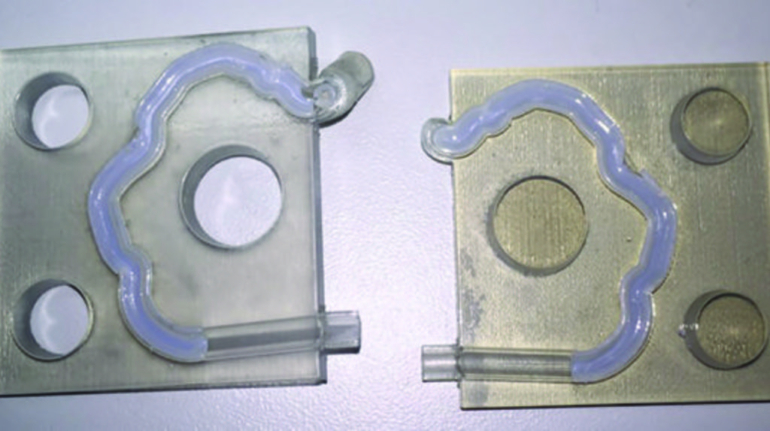
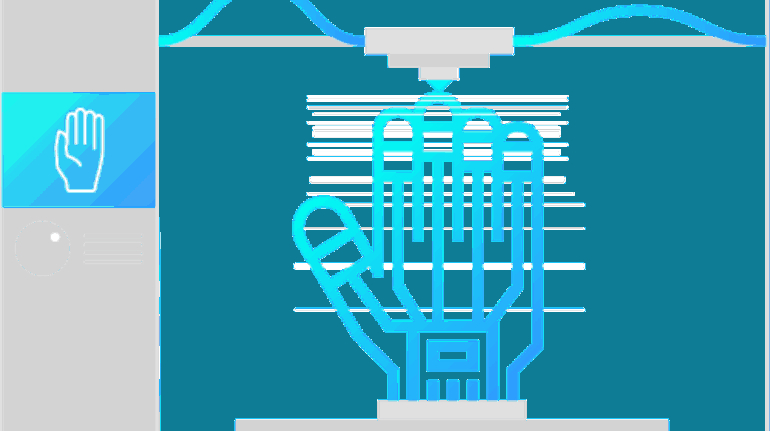
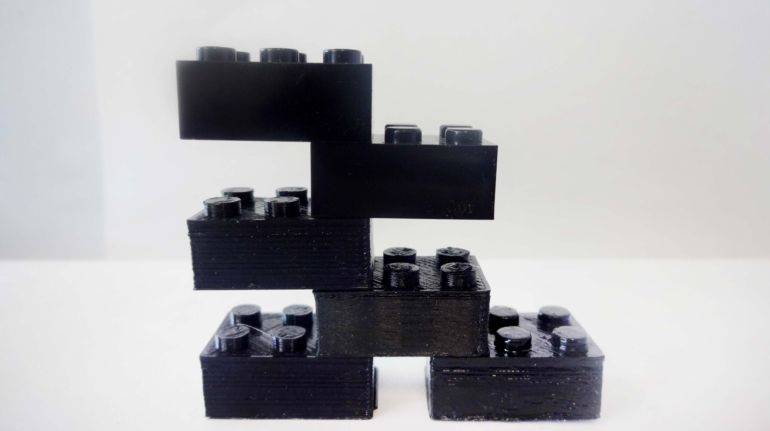
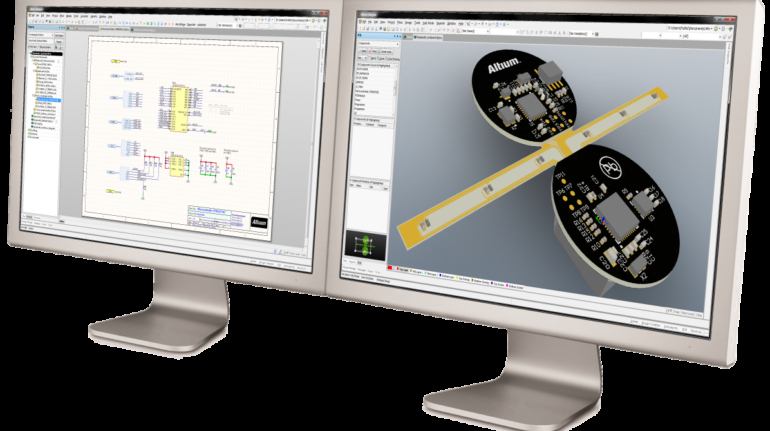
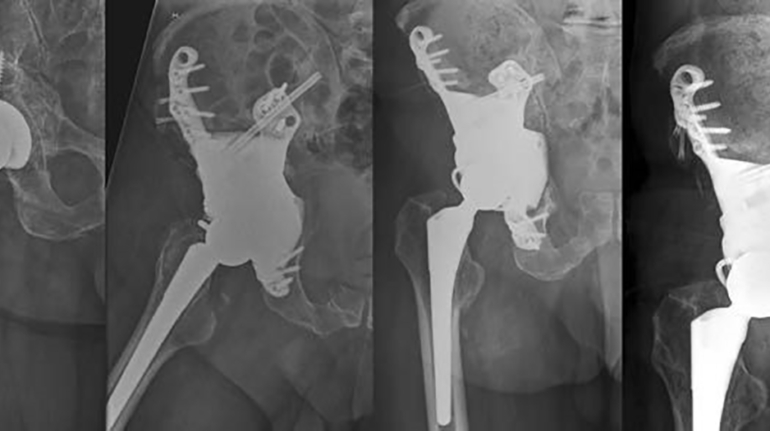
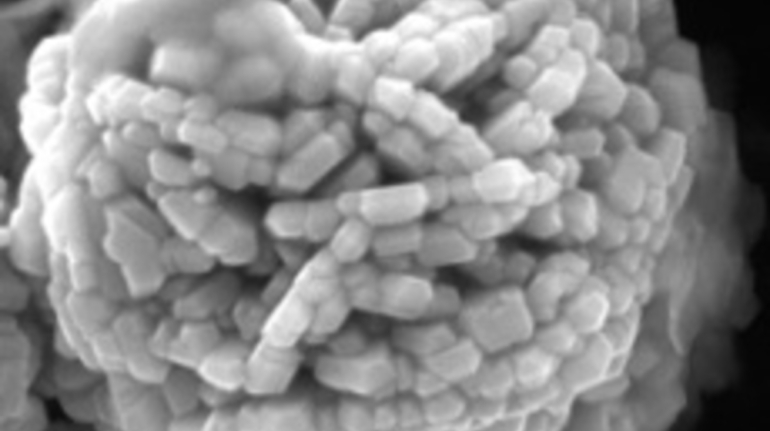
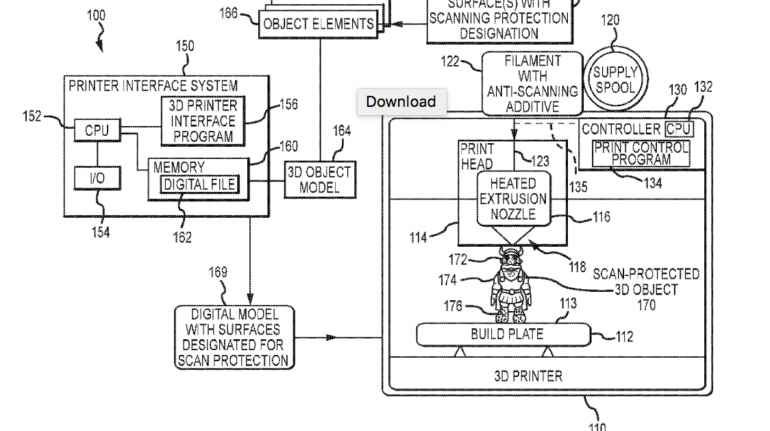
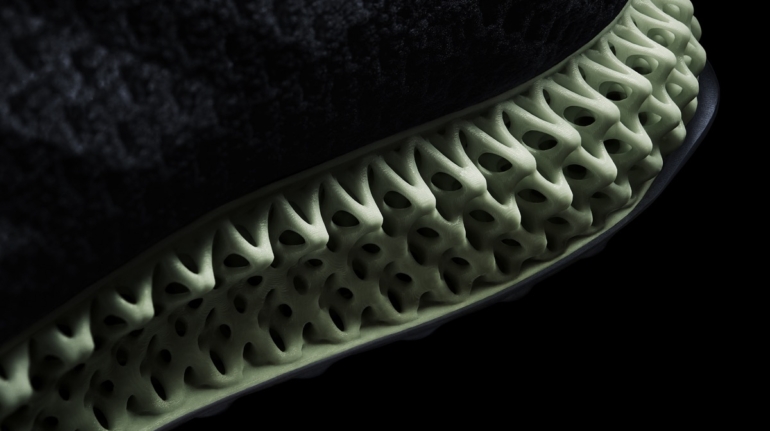
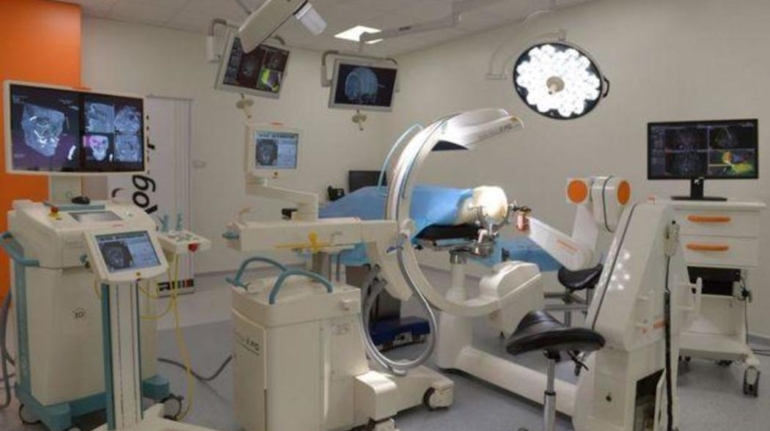
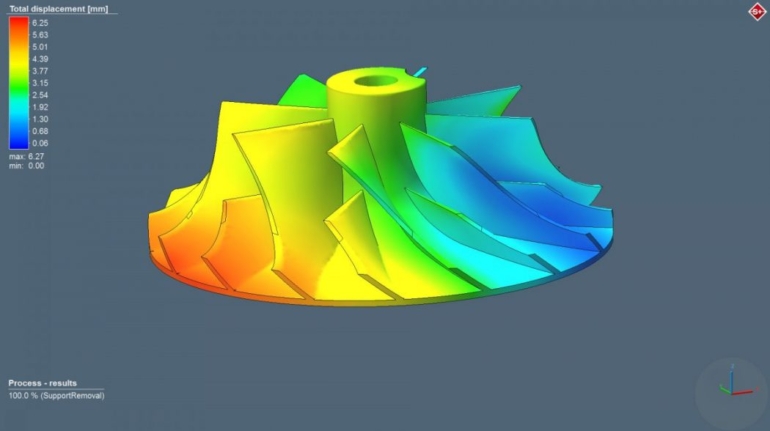
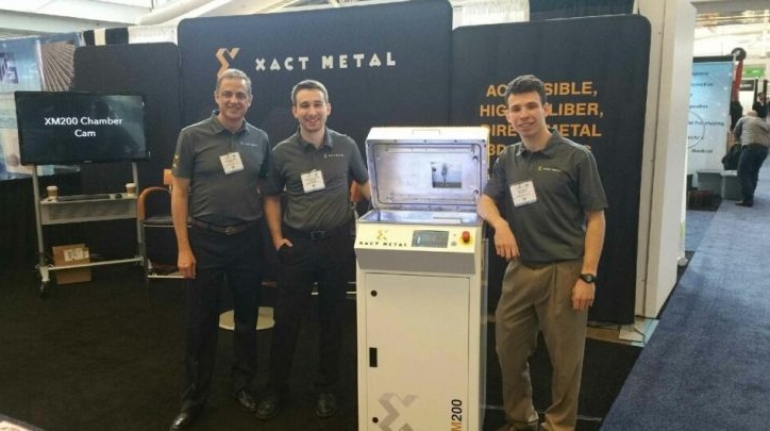
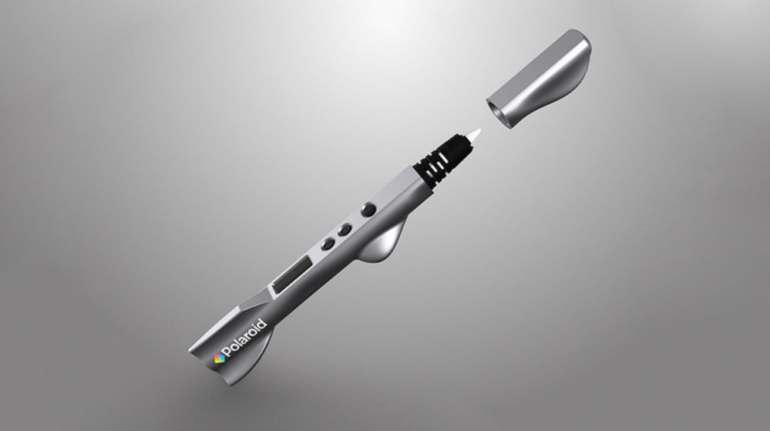
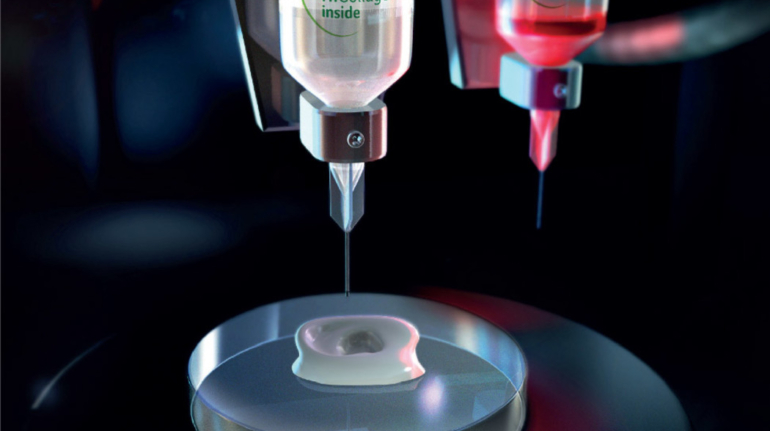
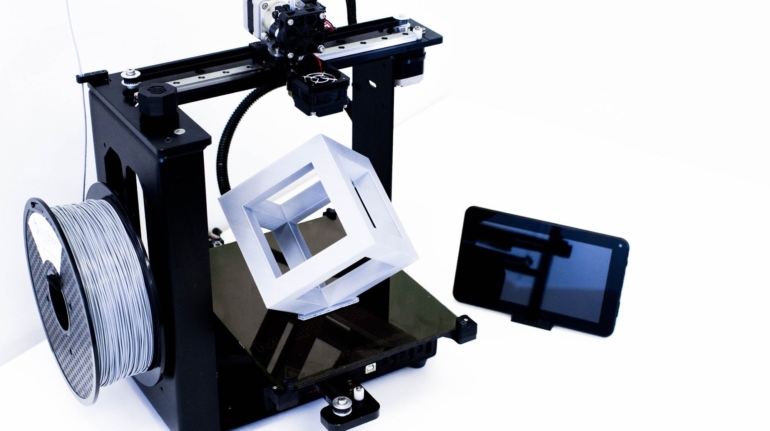
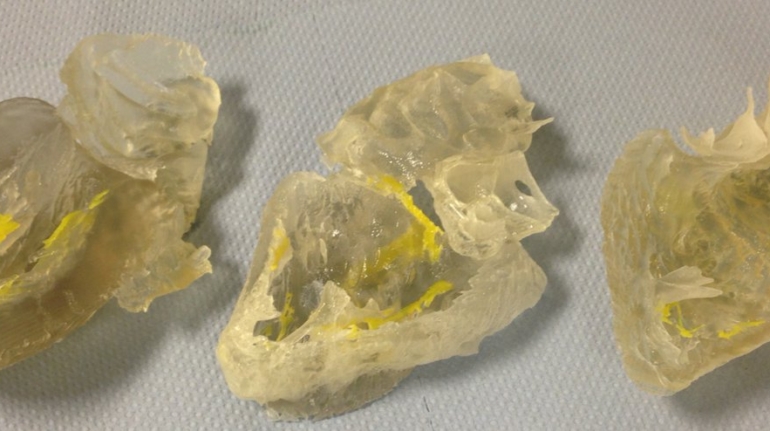
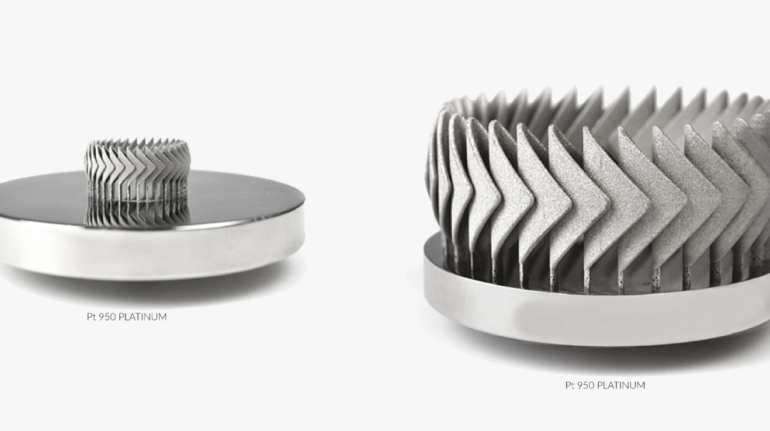
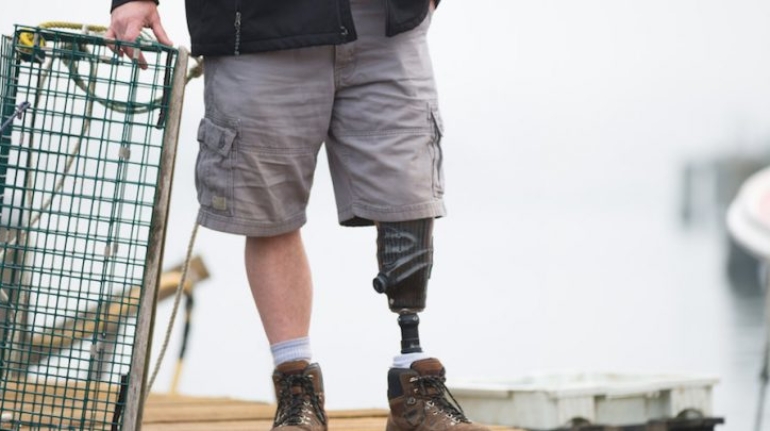
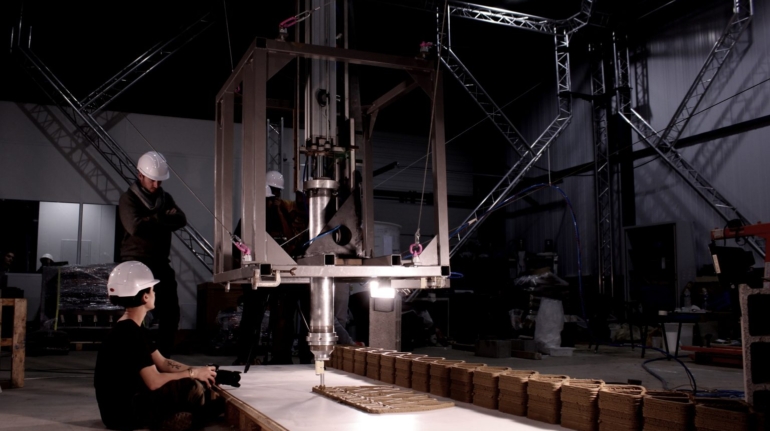
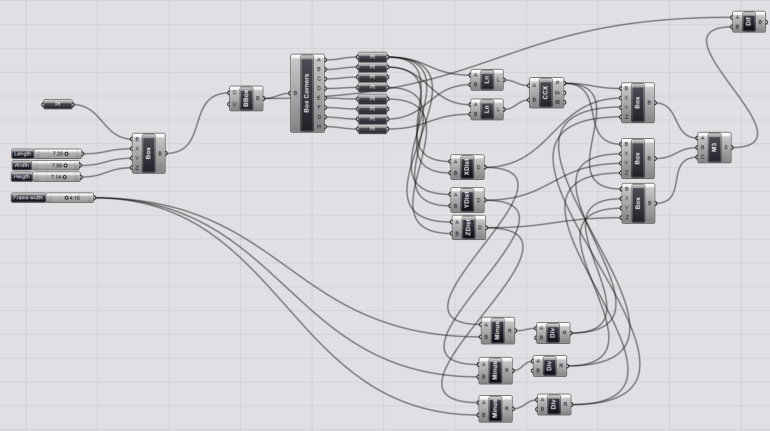
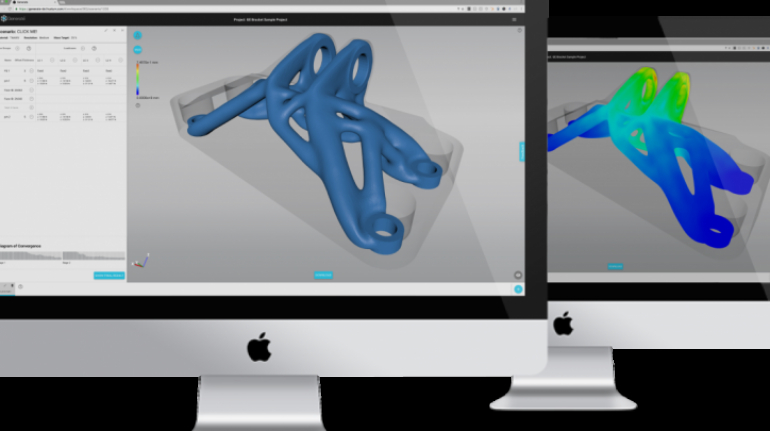
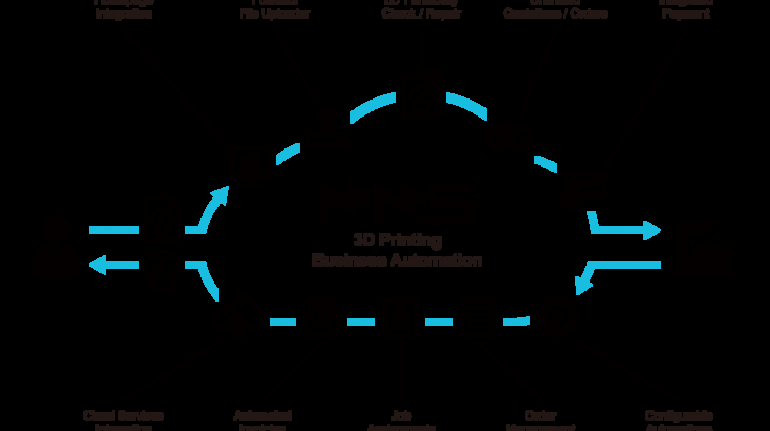
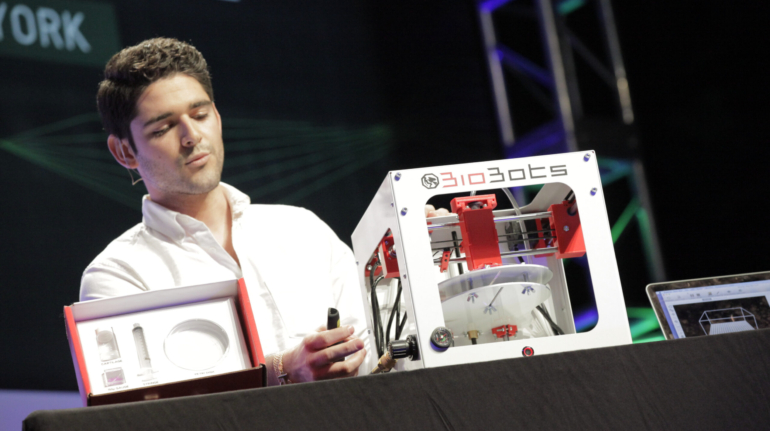
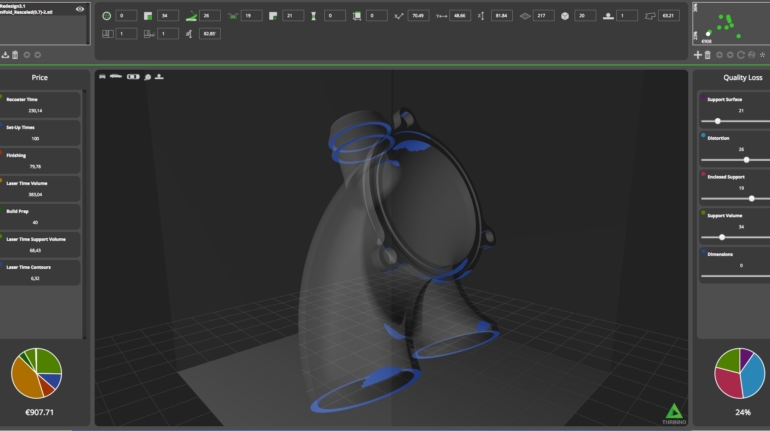
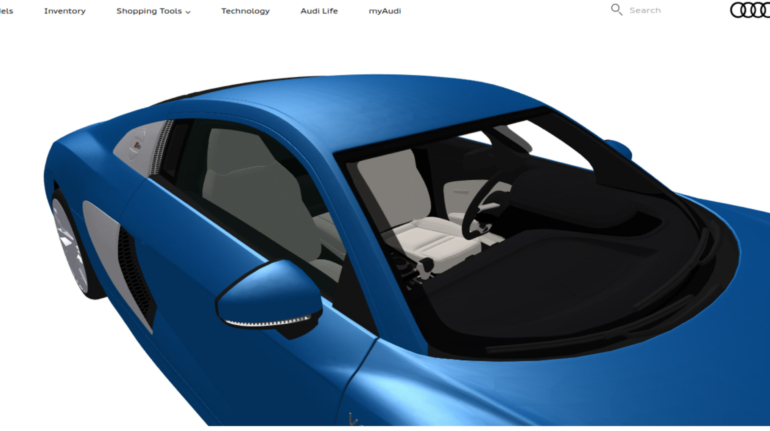
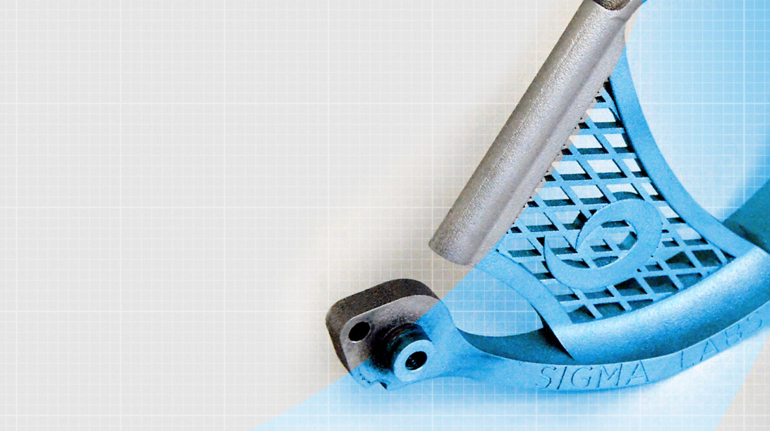
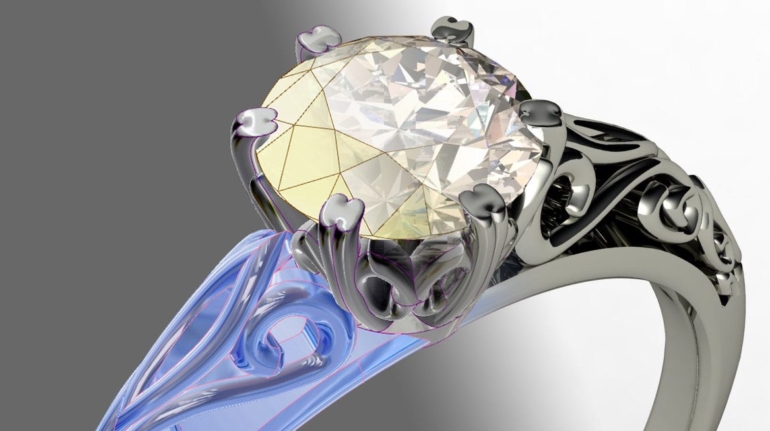
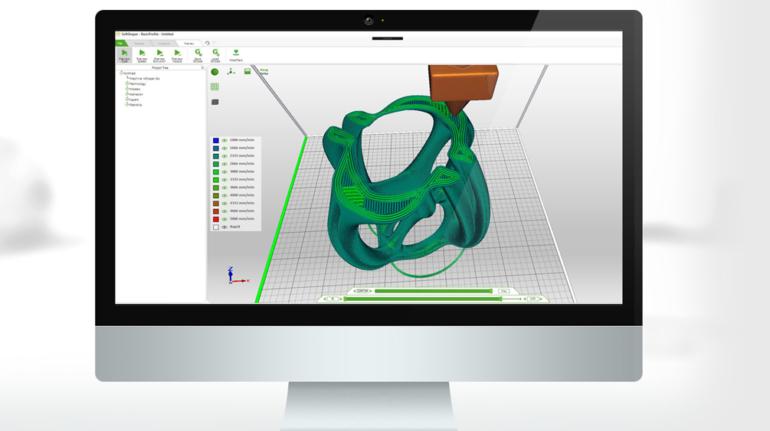
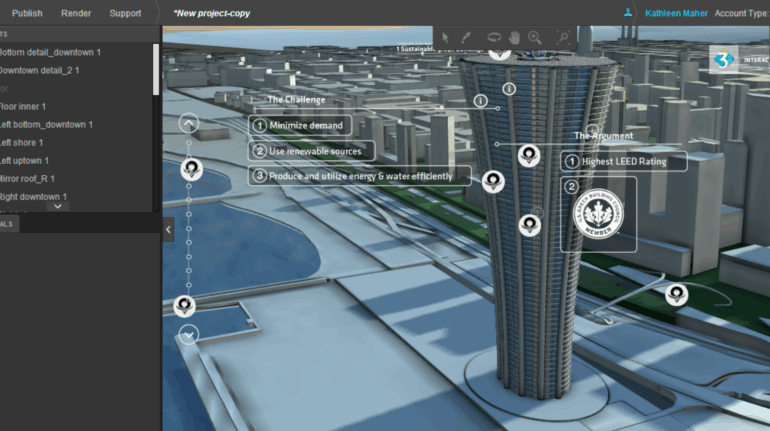
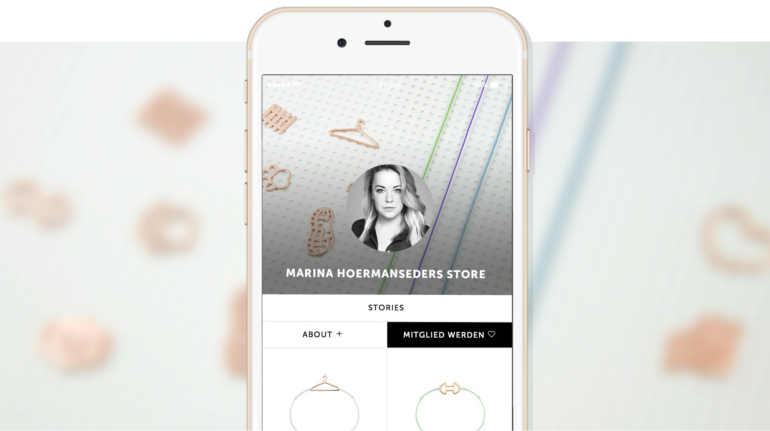
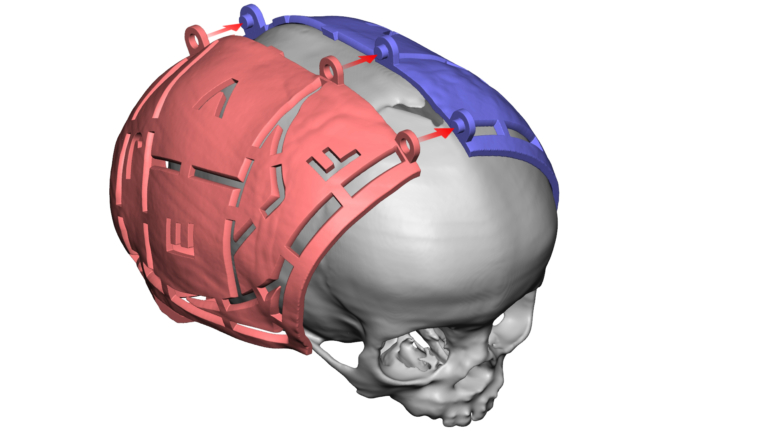
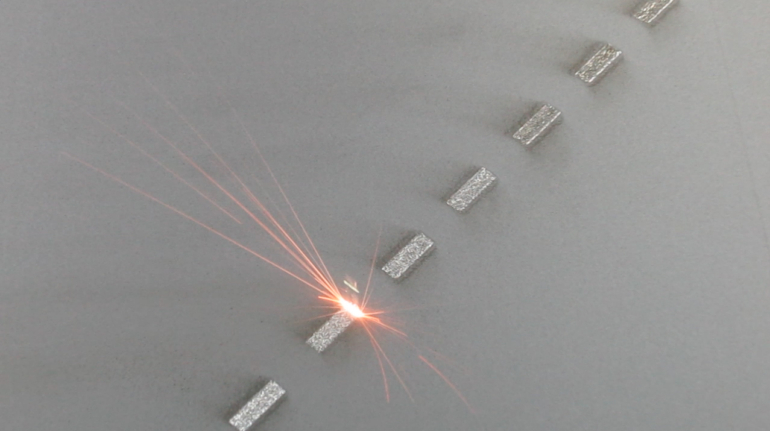
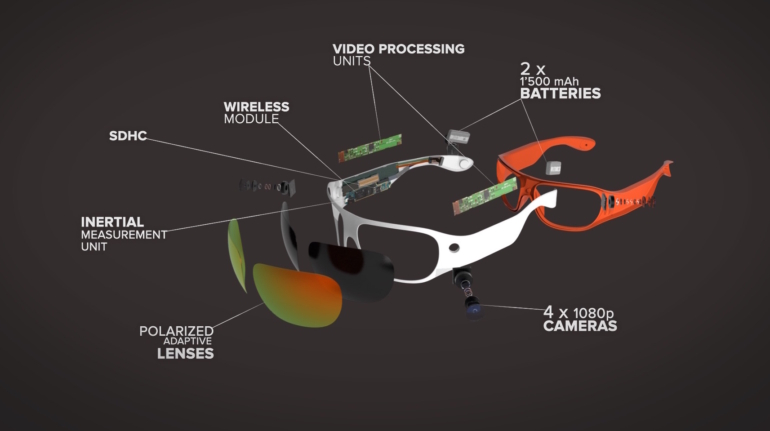
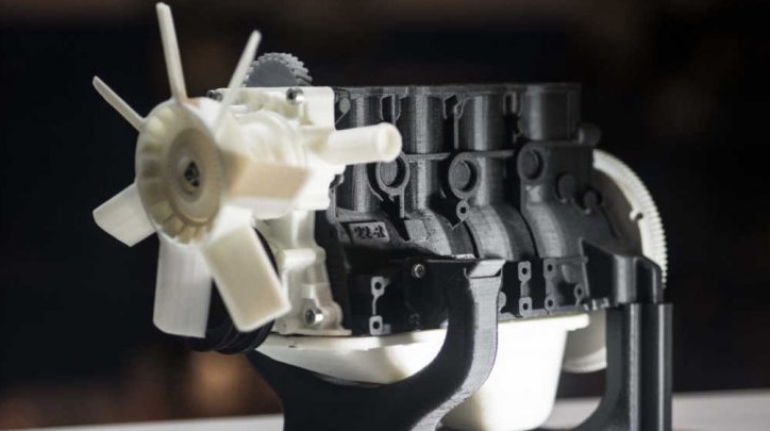
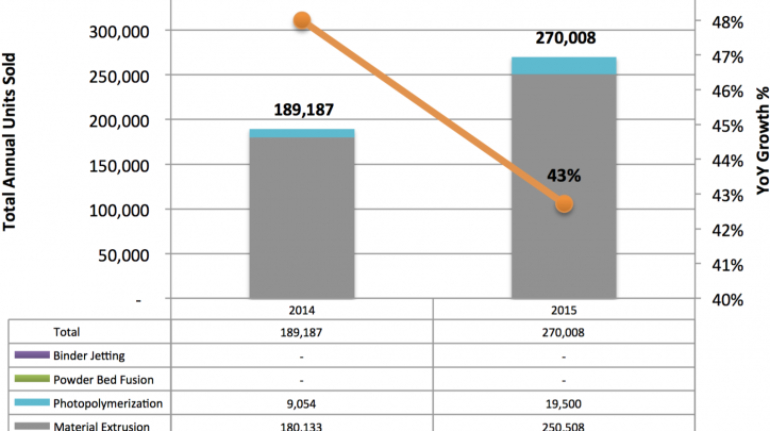
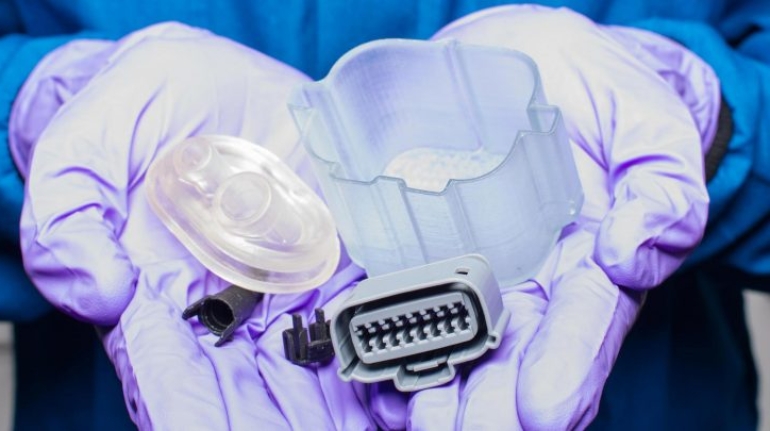
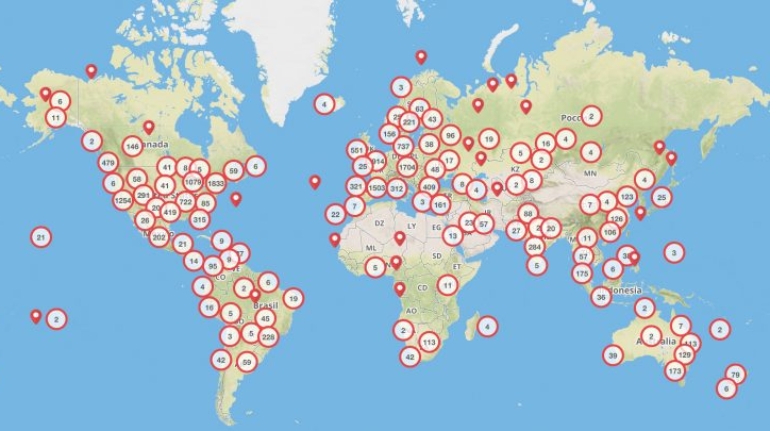
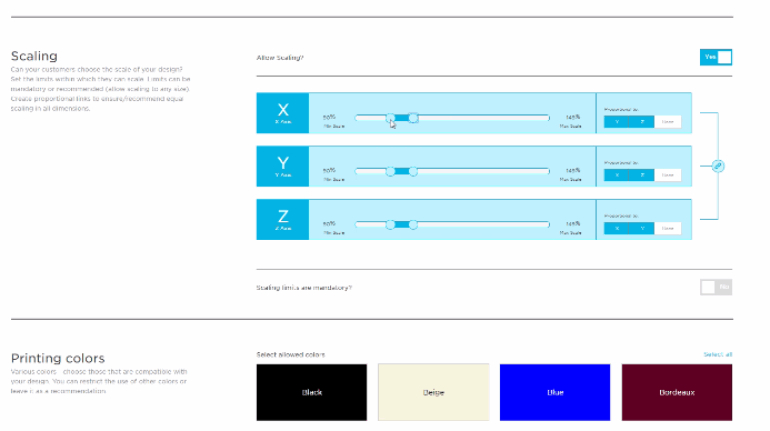
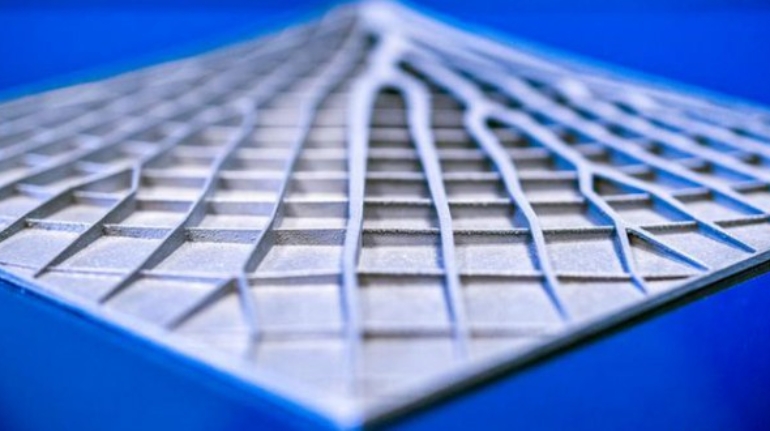
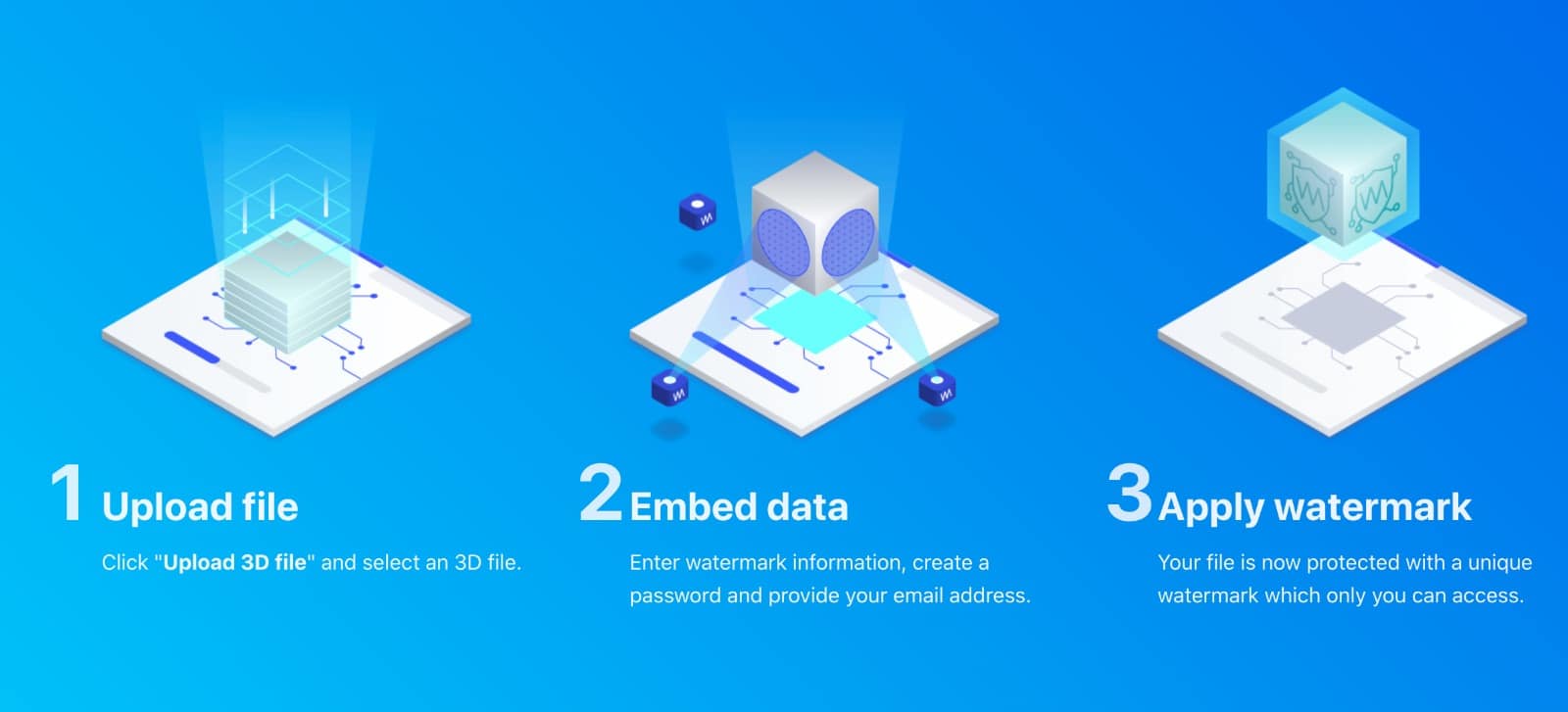
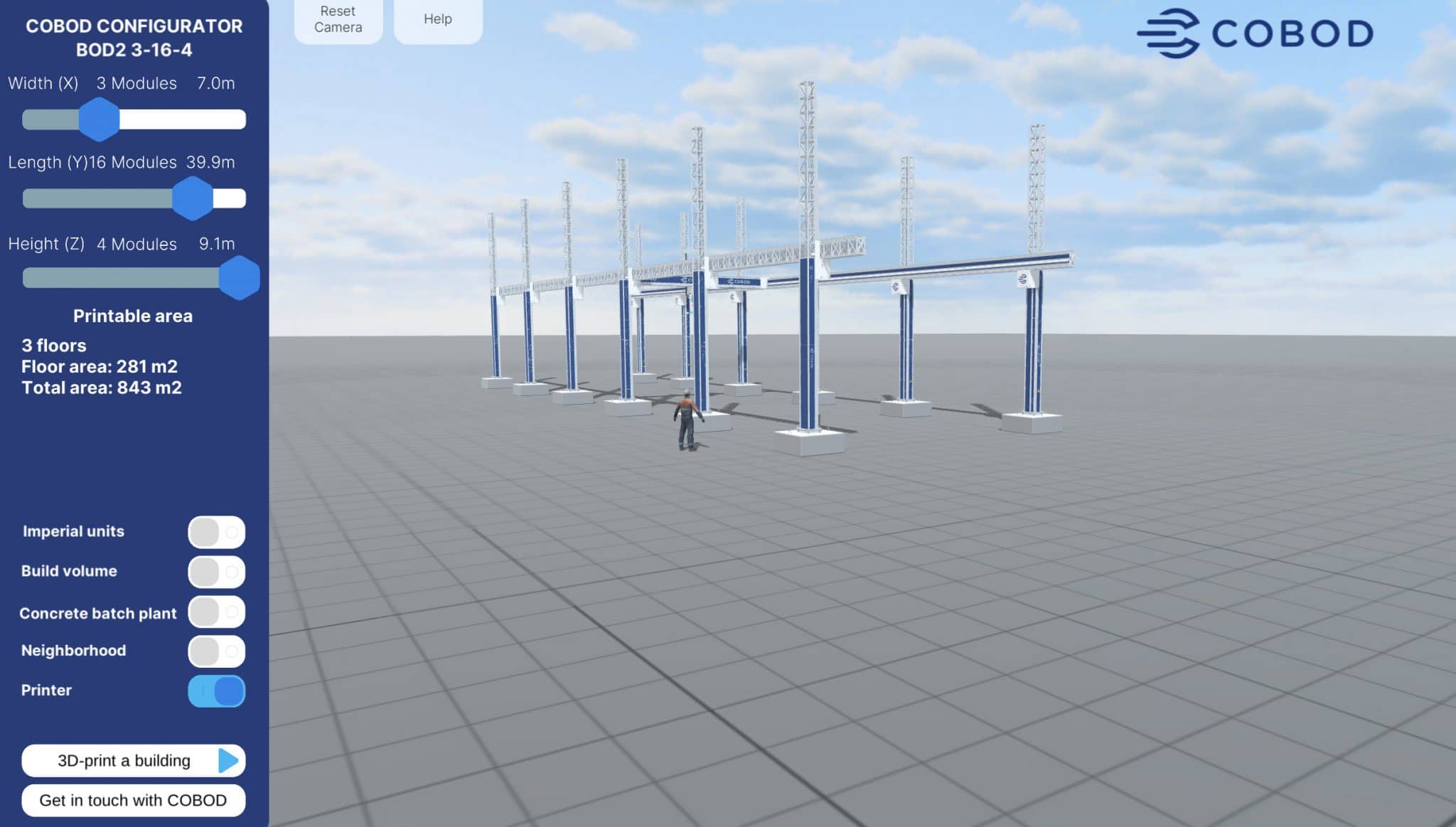
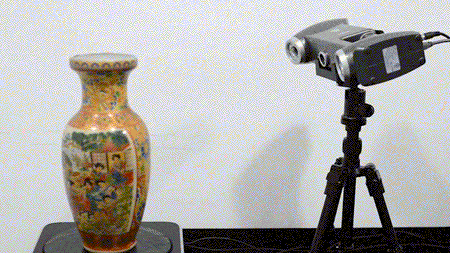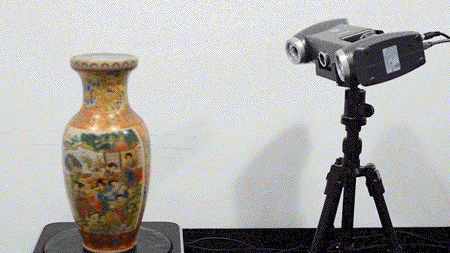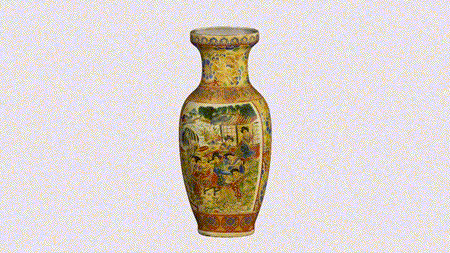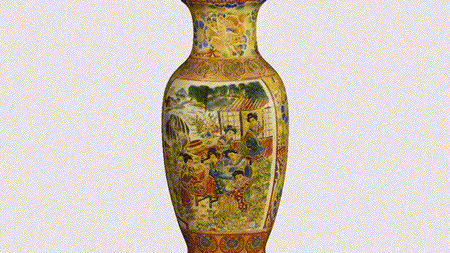
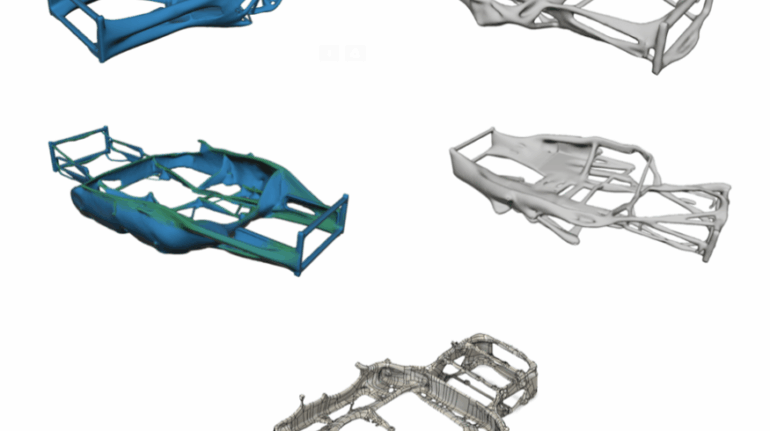
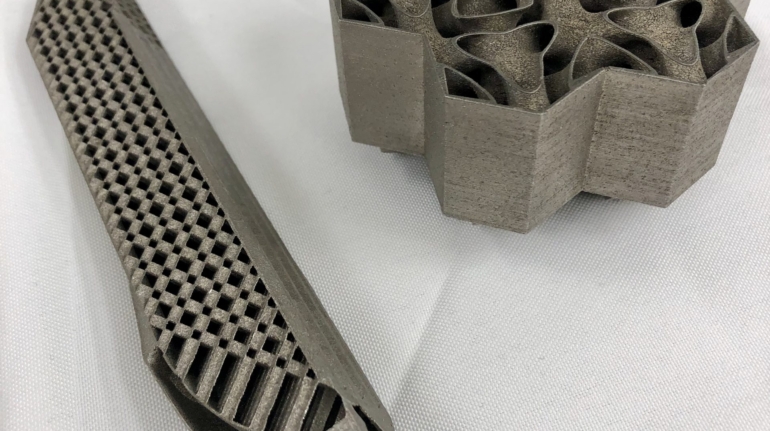
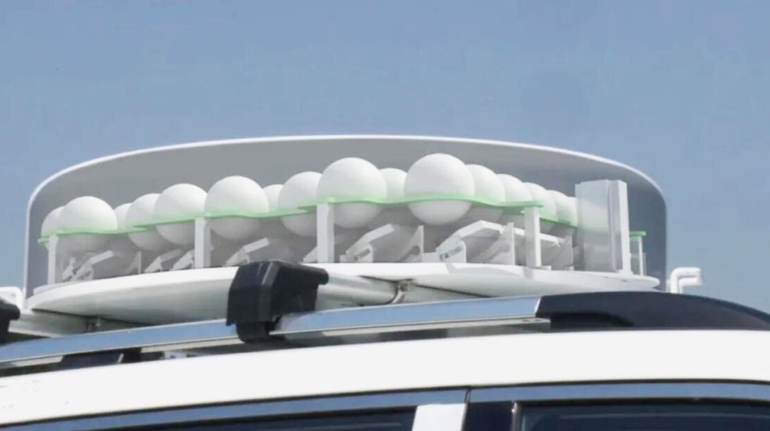
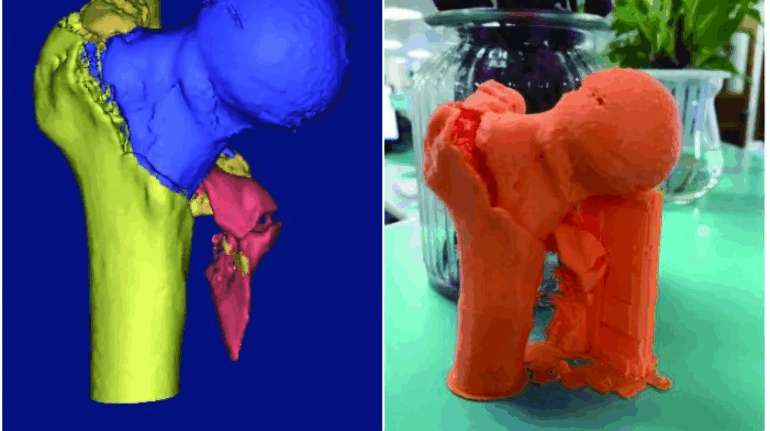
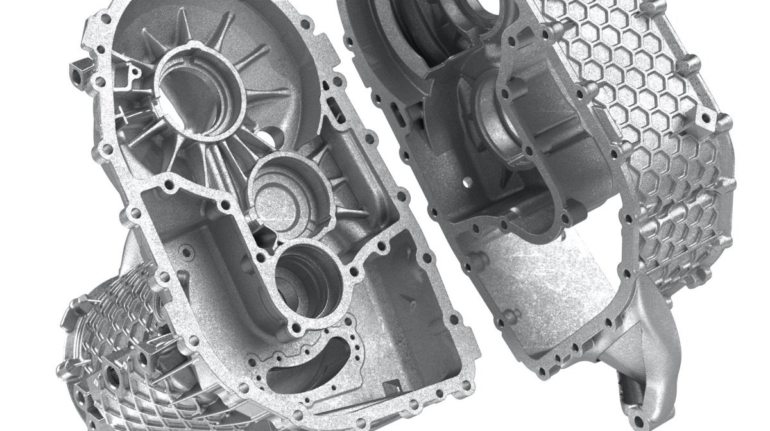
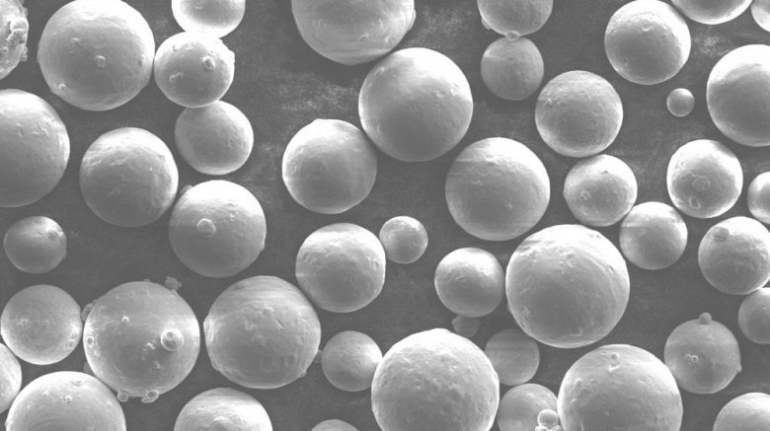
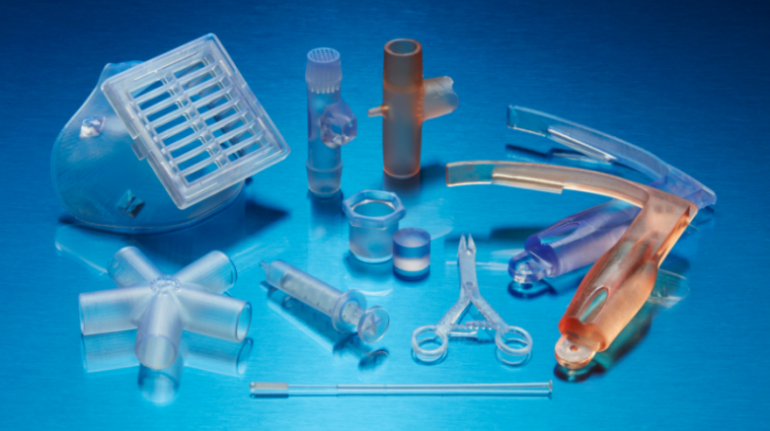
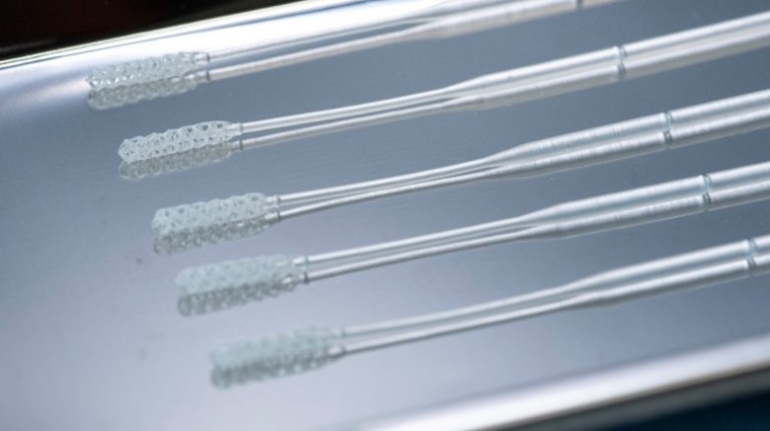
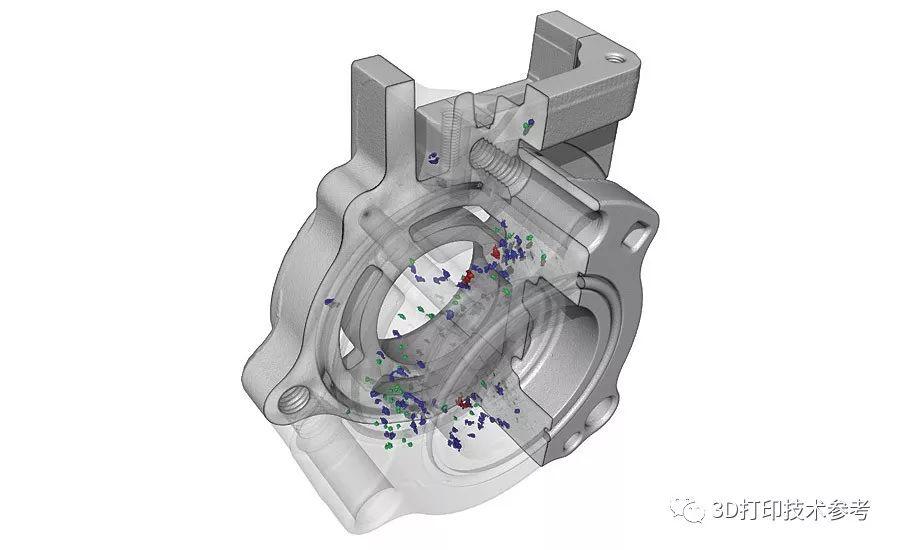
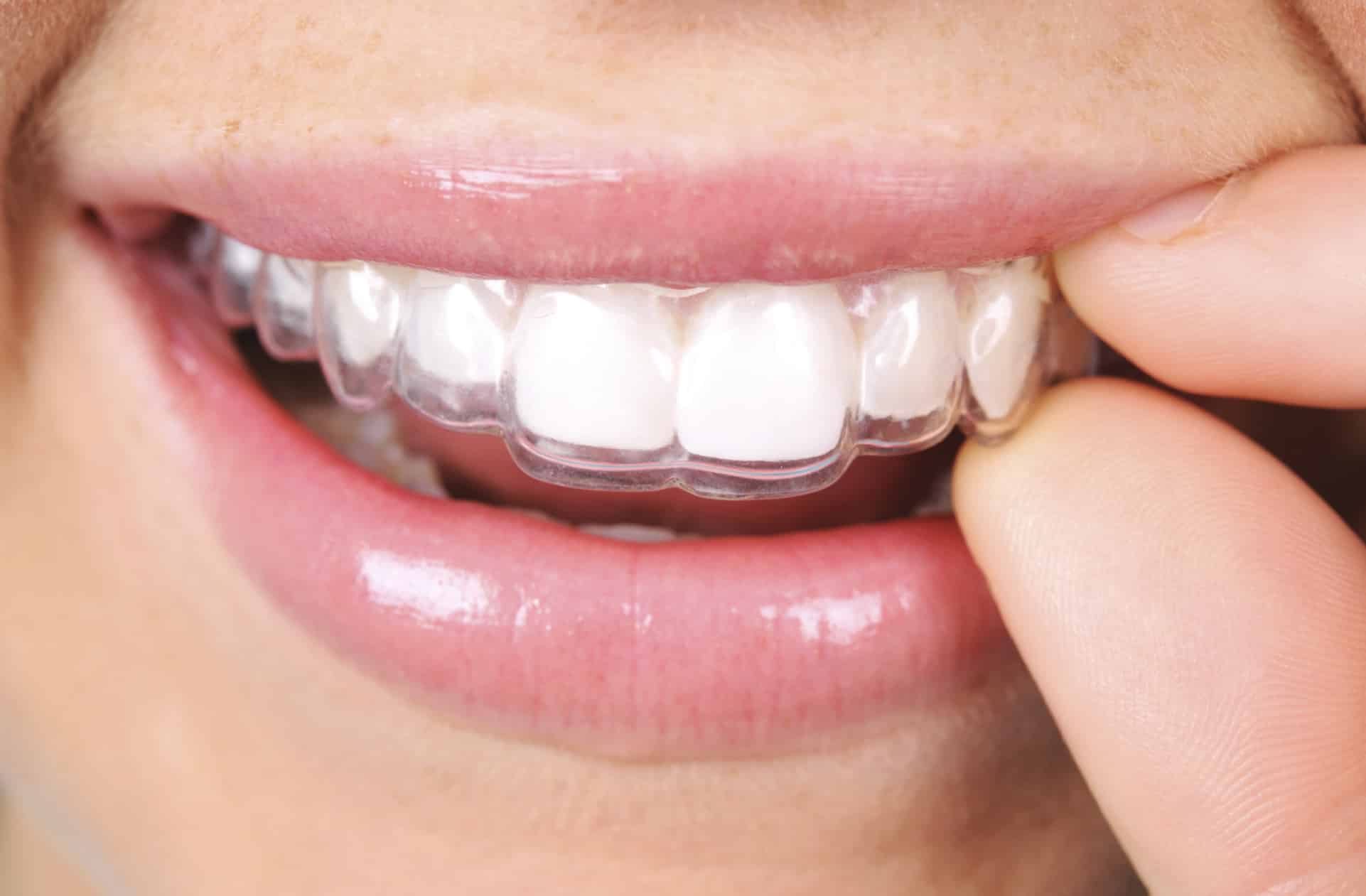
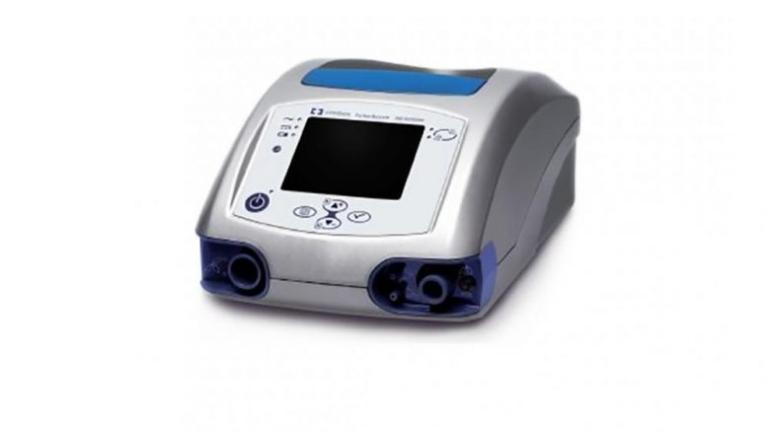
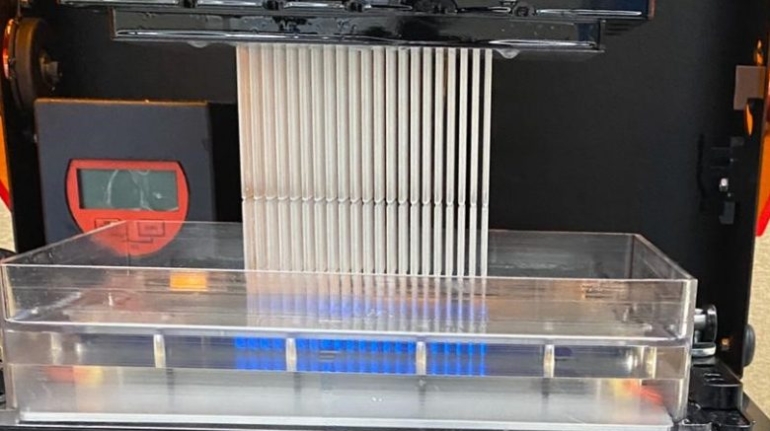
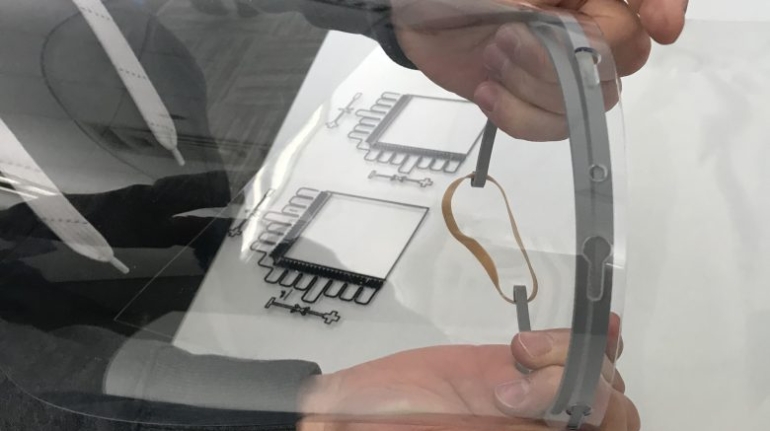
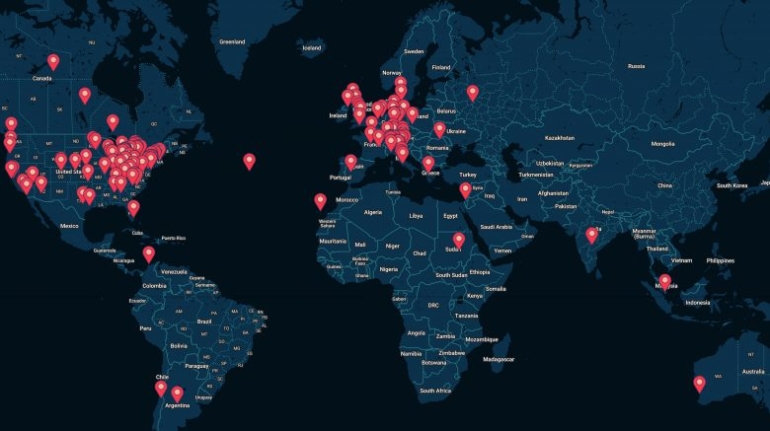
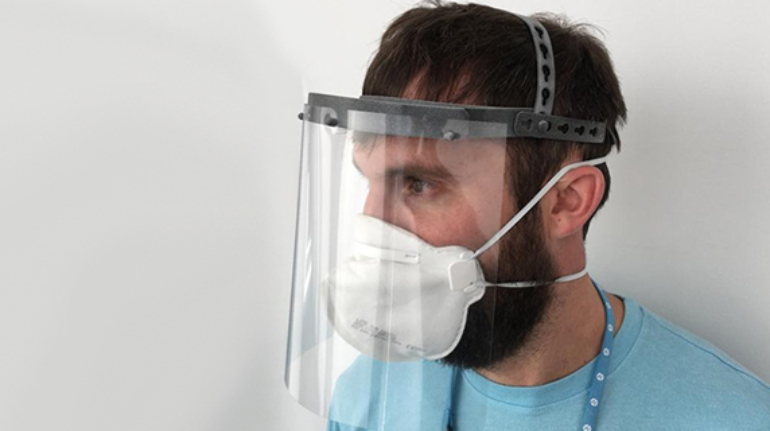
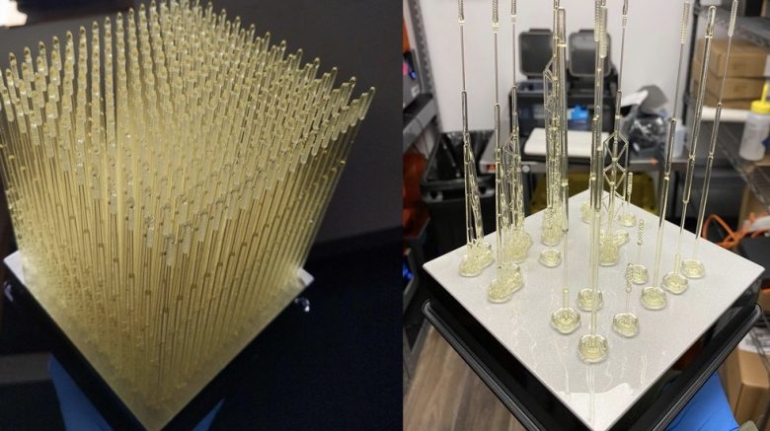
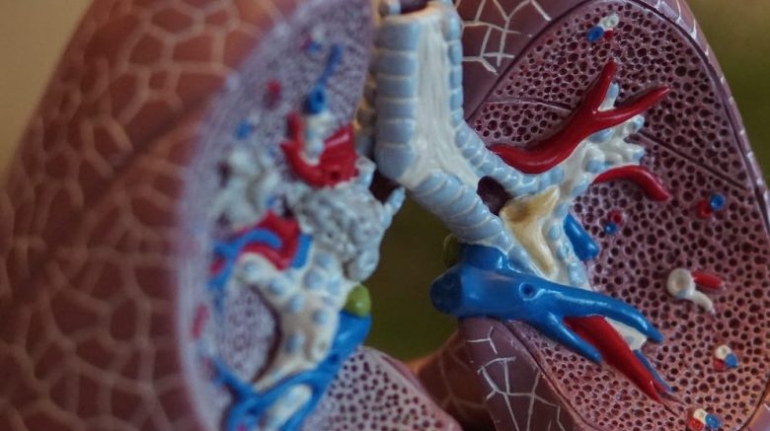
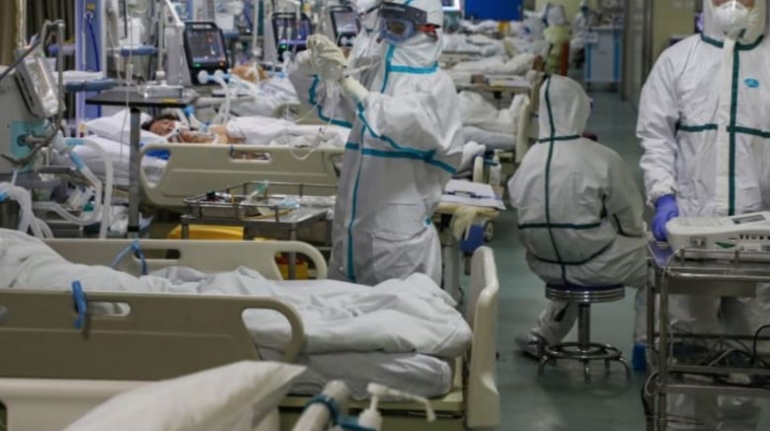
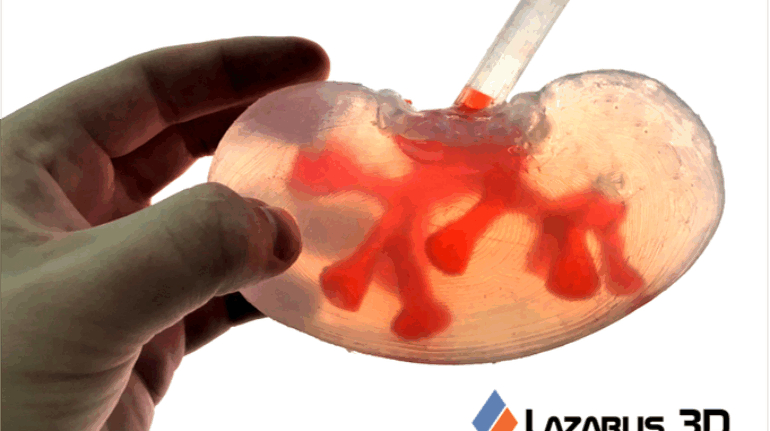
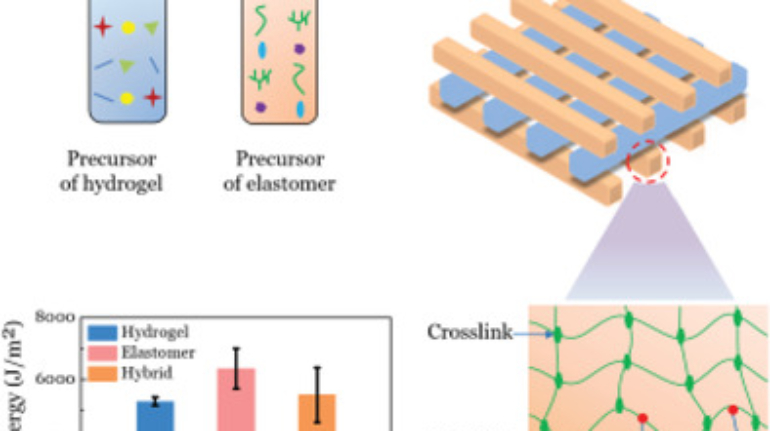
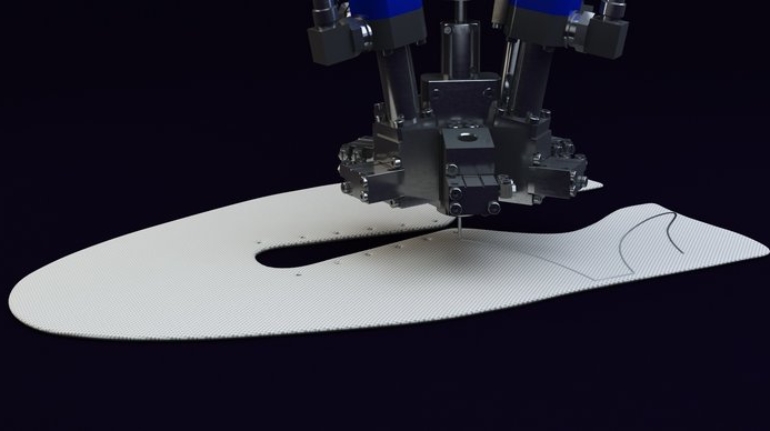
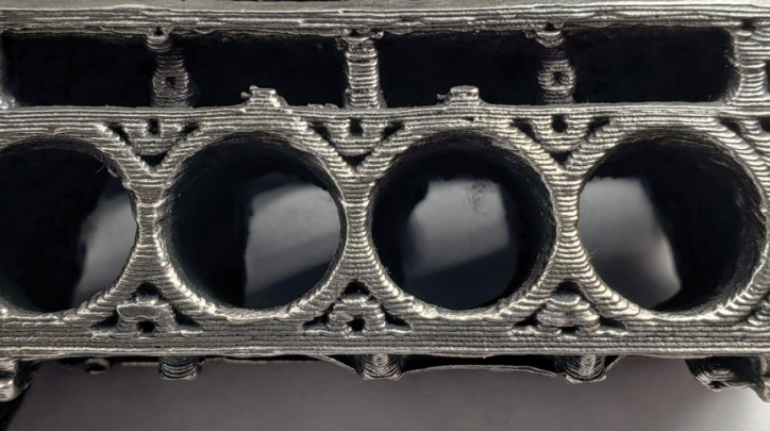
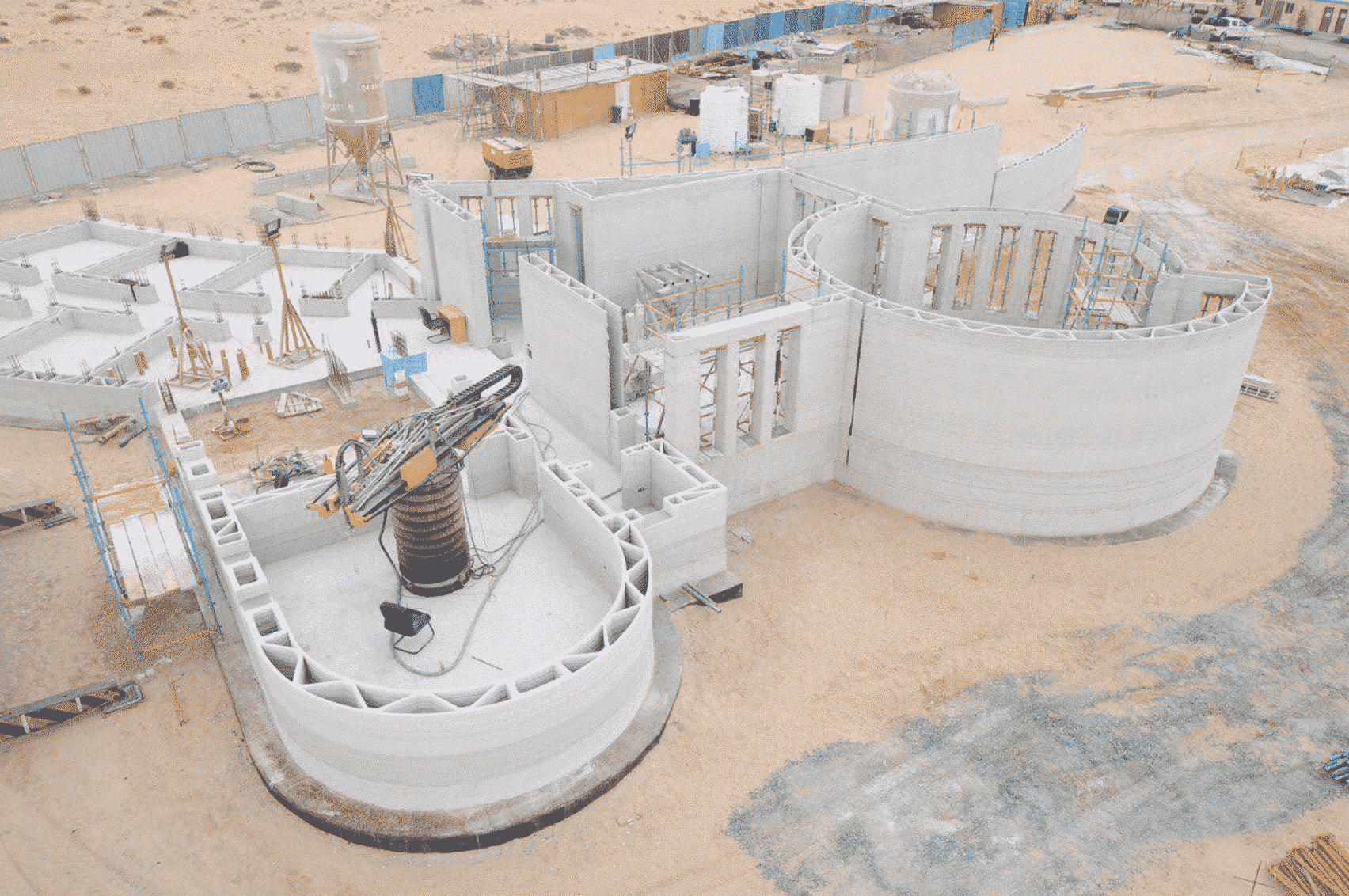
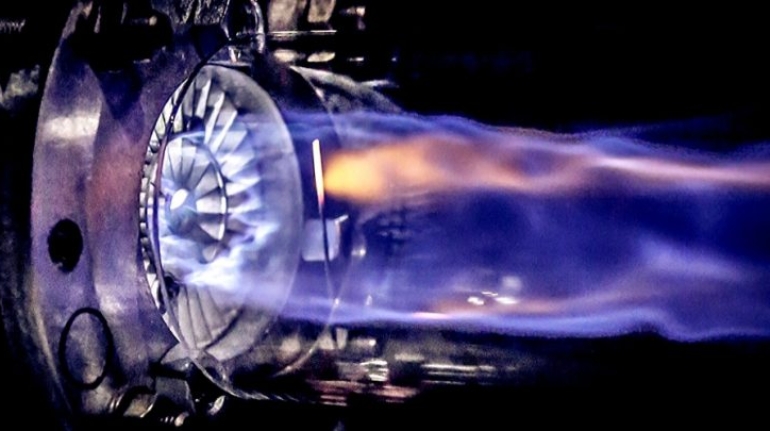
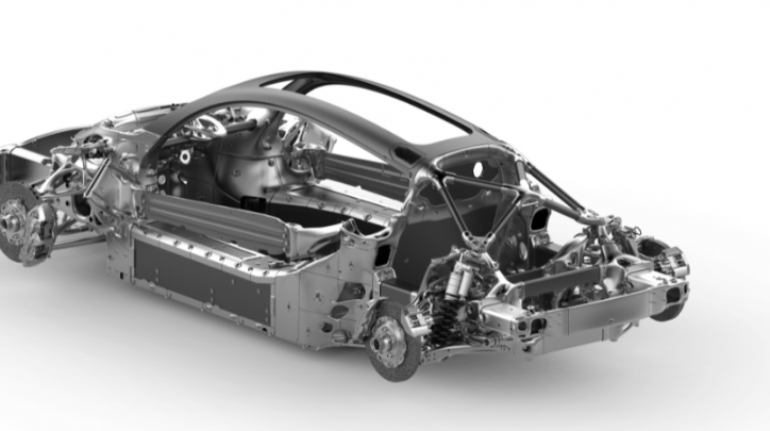
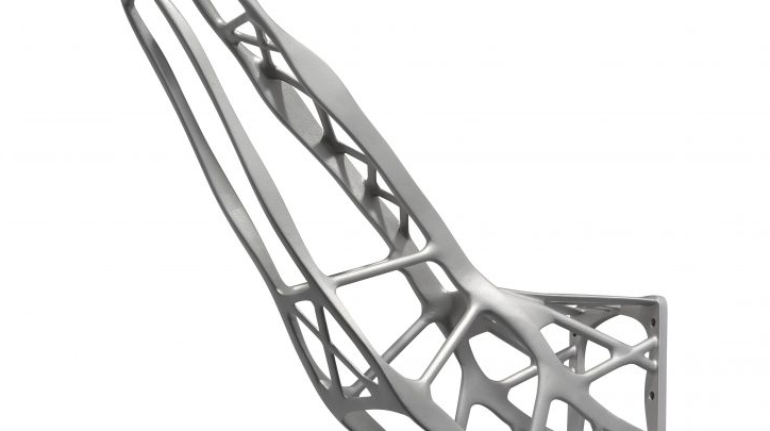
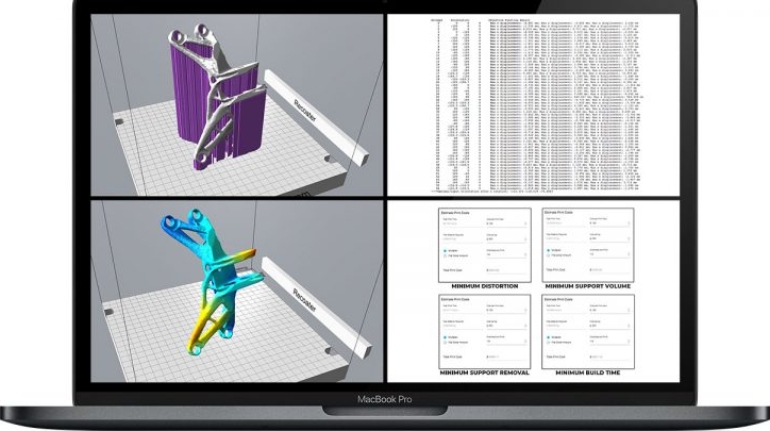
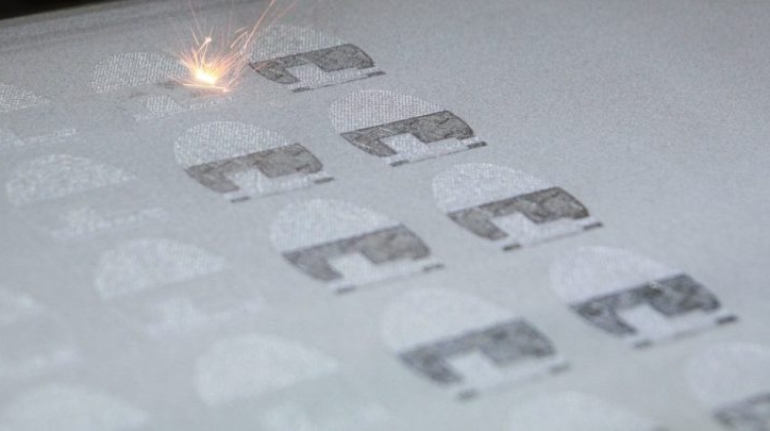
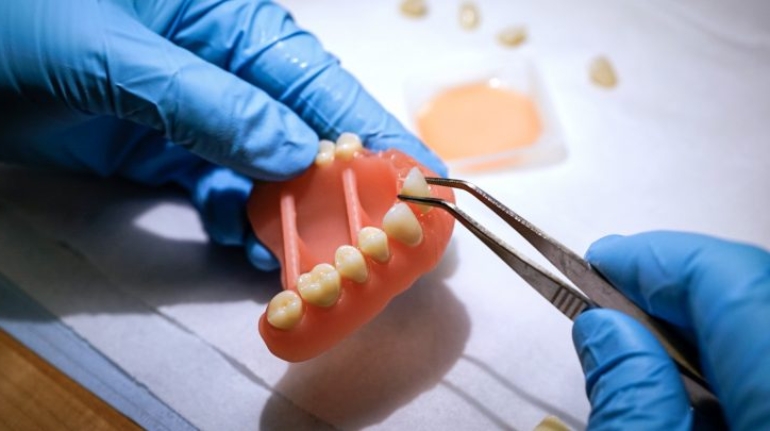
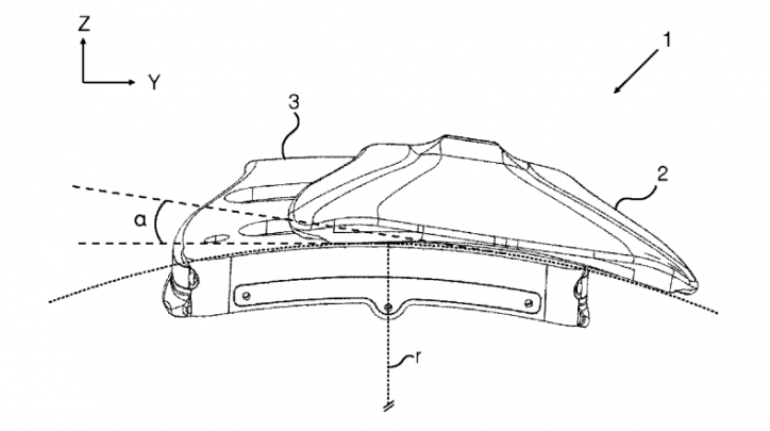
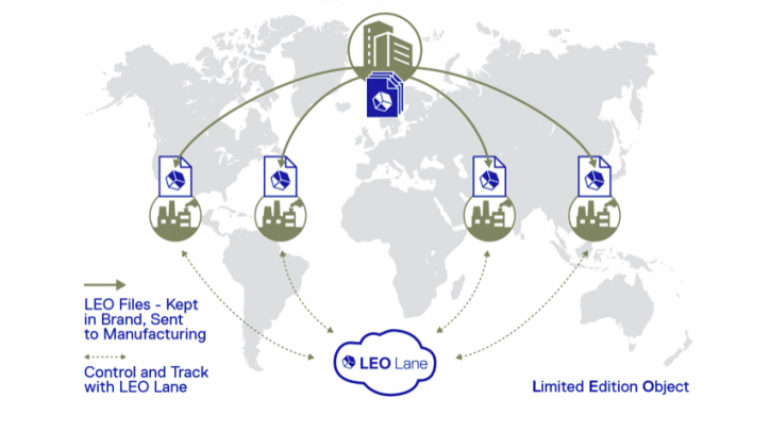
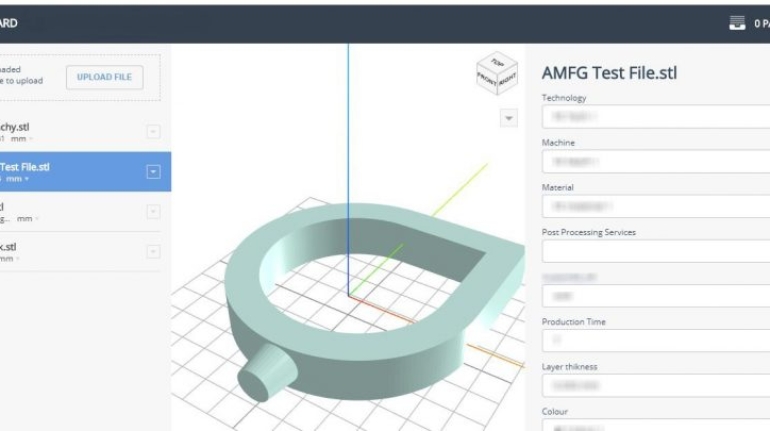
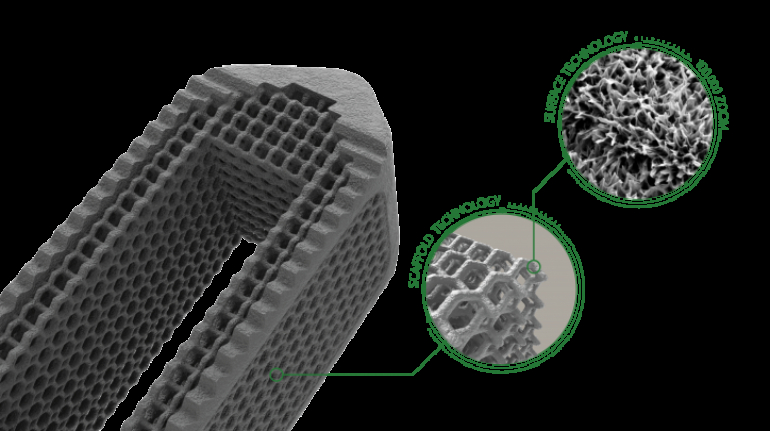
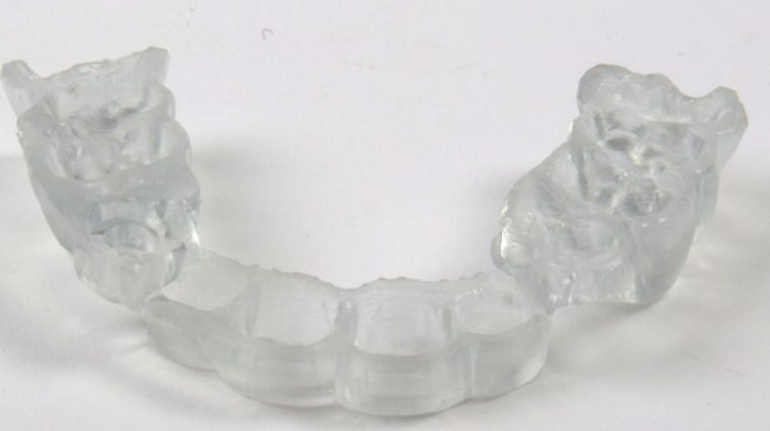
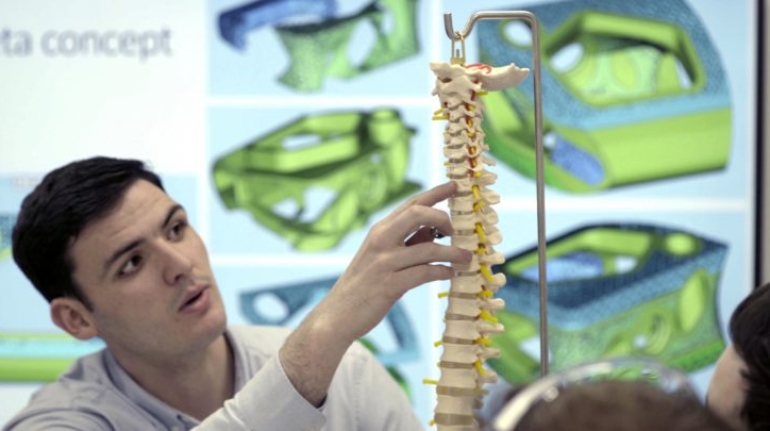
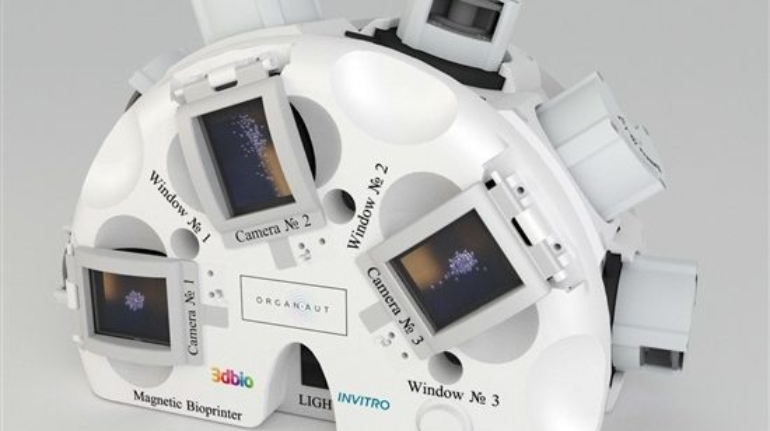
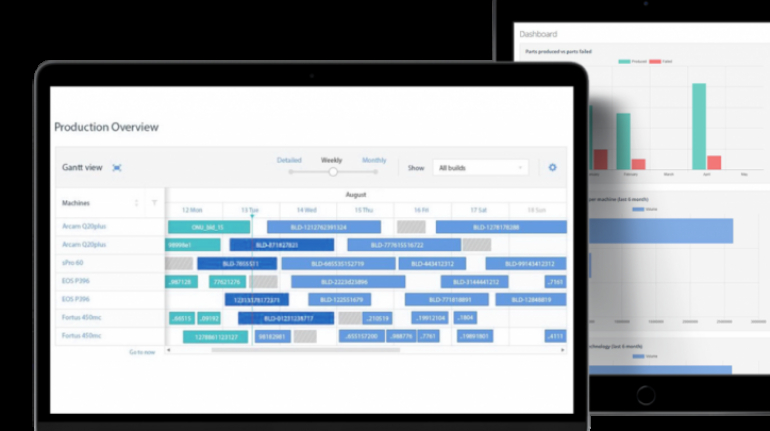
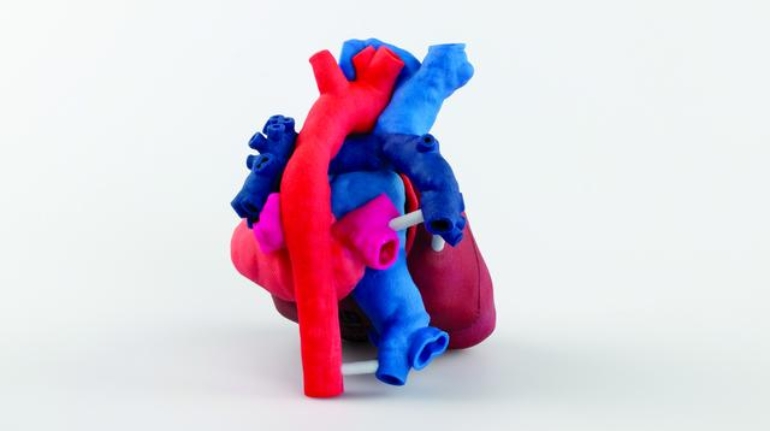
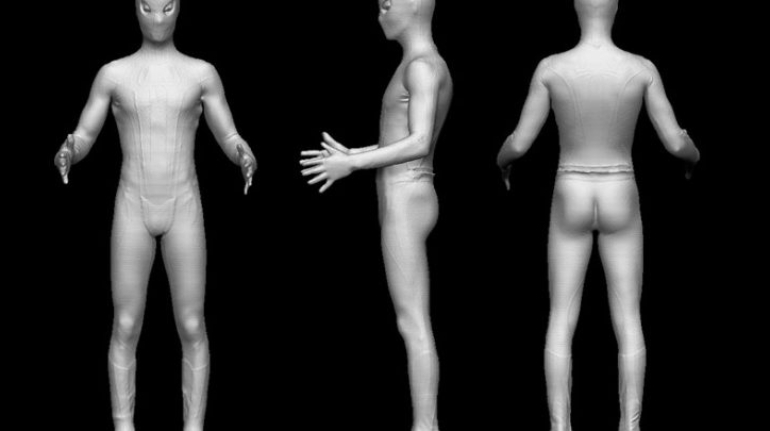
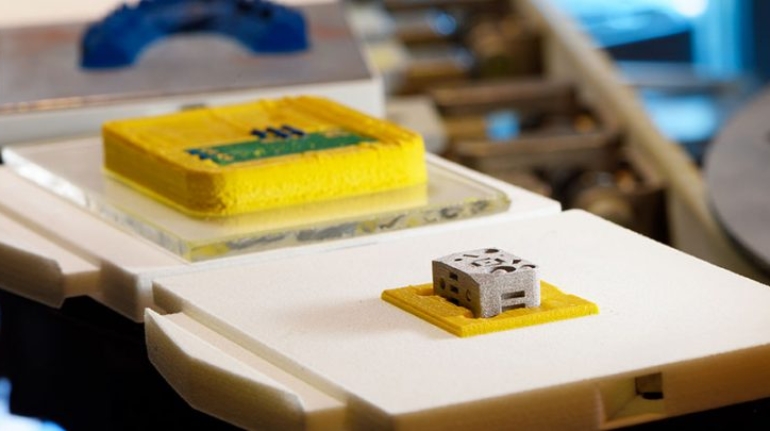
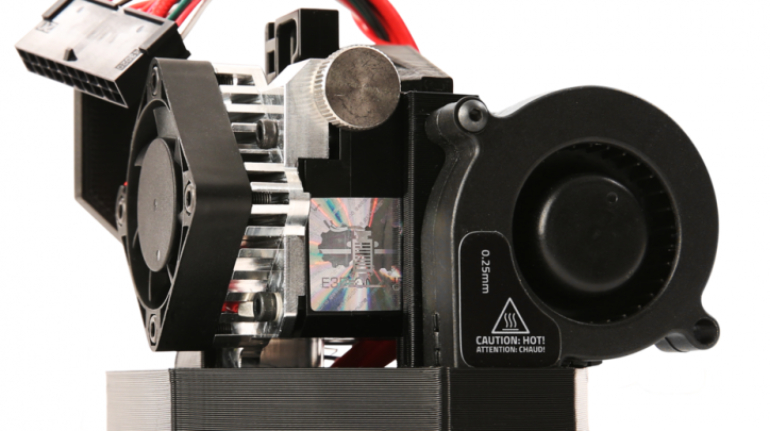
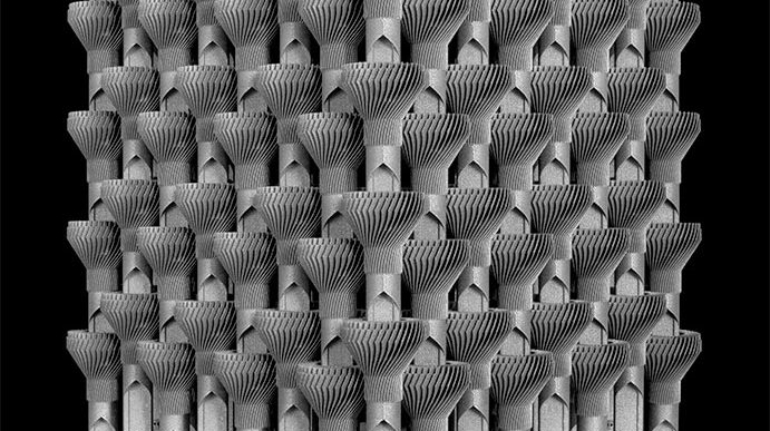
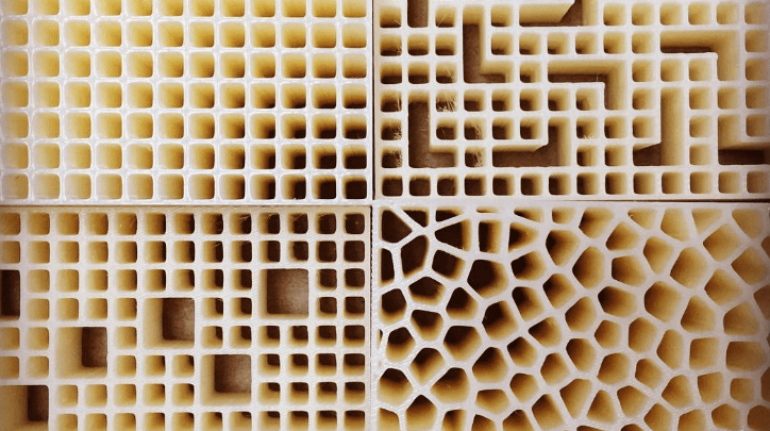
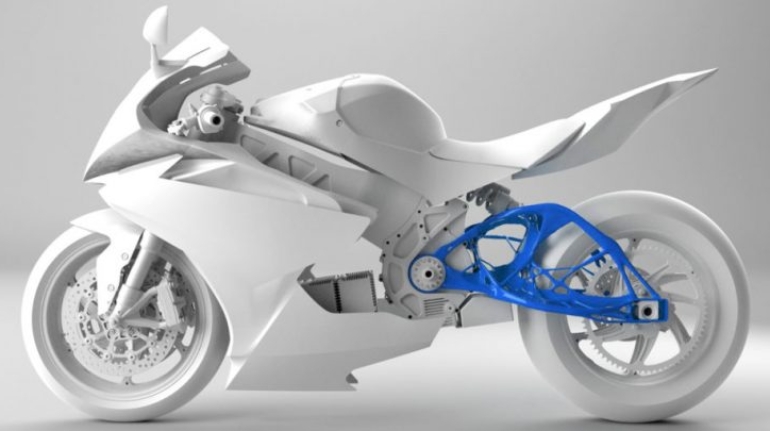
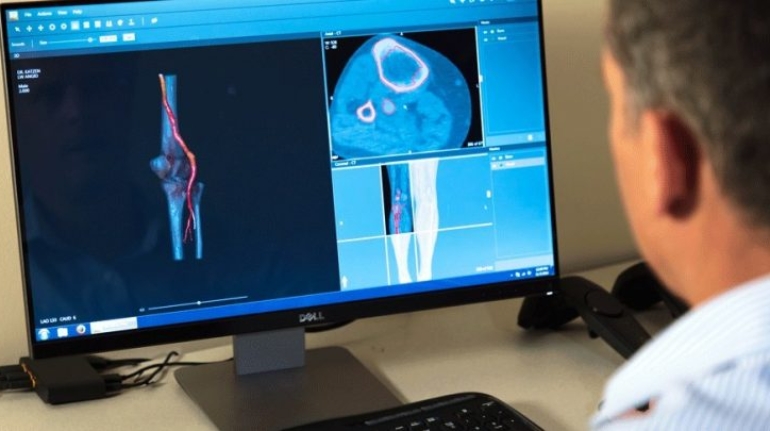
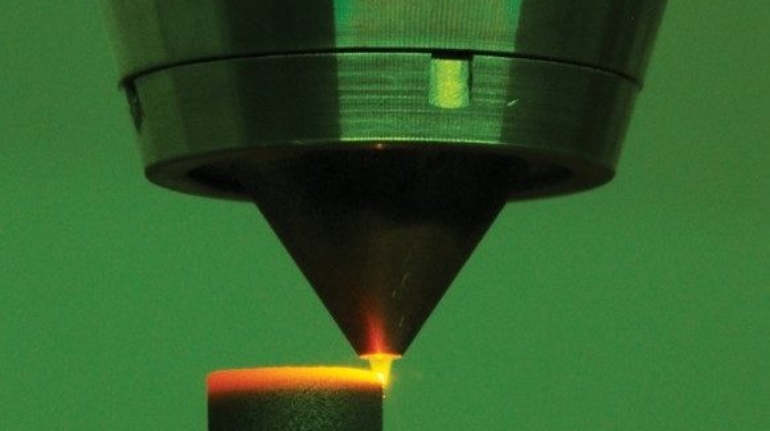
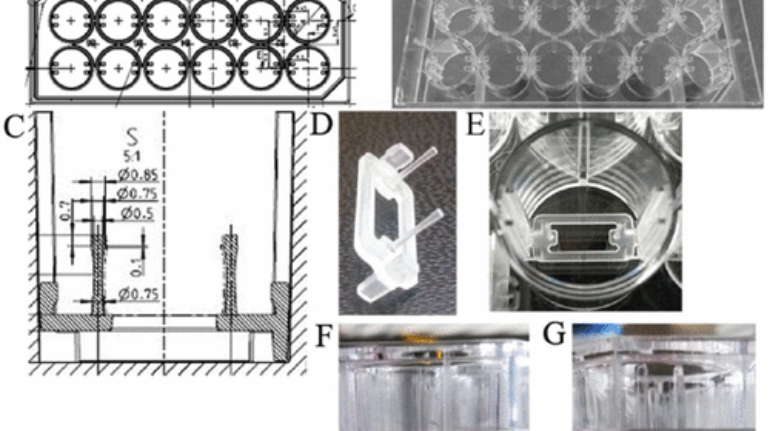
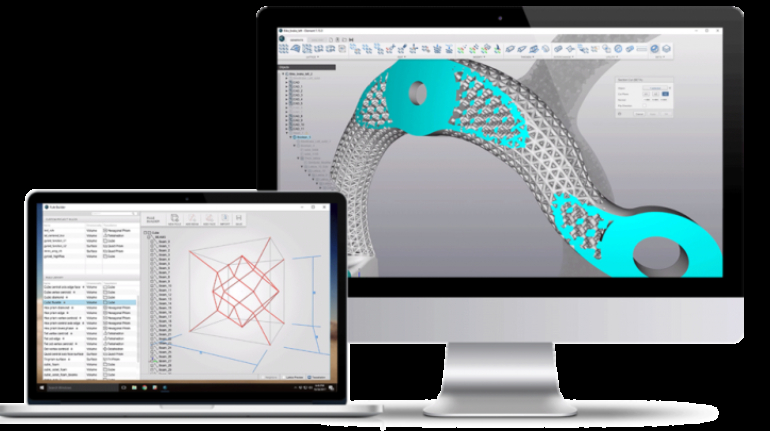
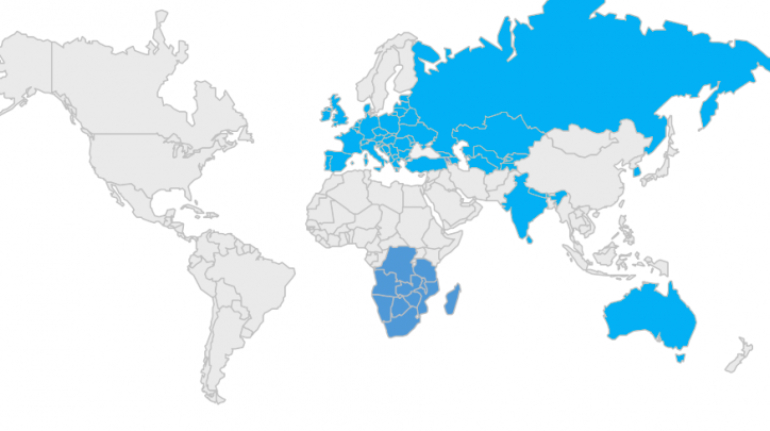
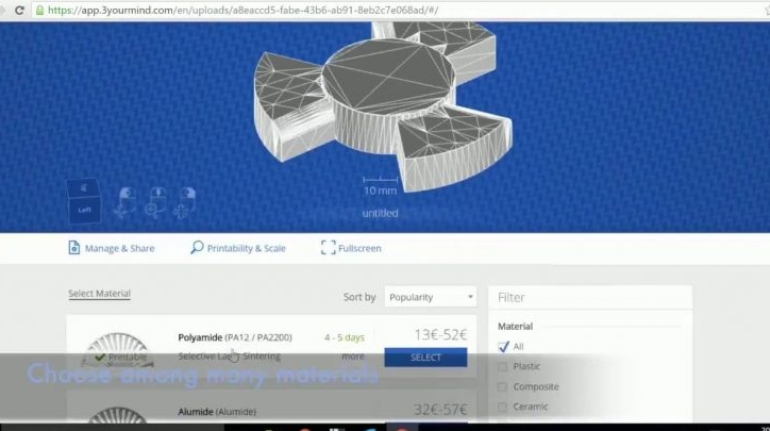
For the first time South Park super fans, technophiles and collectible geeks will be able to purchase some of their favorite South Park characters, previously unavailable in physical form, thanks to Source3. Source3, a startup founded by former Google and music industry executives specializing in digital content management, has launched a collector-inspired line of South Park characters presented in full-color 3D prints, featuring year-round introductions of new and old characters. South Park creators Matt Stone and Trey Parker have hand-signed a limited number of Stan, Kyle, Kenny and Cartman figurines which select fans who purchase the full set on Shapeways will have an opportunity to win (see the store links for official rules). The product lineup also includes fan favorites like Candidate Garrison, Terrance and Phillip, Tweek and Clyde Frog. The Source3 team is thrilled to celebrate the 20th season of South Park in partnership with Viacom, South Park Studios, Brandgenuity, WhiteClouds, Amazon, and Shapeways. The goal of Source3 is to help large content distribution websites, such as popular 3D printing marketplaces like Shapeways, but also consumer product giants like Amazon, to meet the real needs of designers. “We started developing the platform and launching a private beta testing phase first of all for us to develop a better product,” says Source3’s Director of Marketing Tom Simon, whom I finally caught up with, in spite of the 10-hour time zone difference between us. “We also did it for us to be able to go back to potential distribution partners and say to them ‘look, I know your API doesn’t do this today but designers are asking for it, se we can help you develop that part of the API in order to meet the designers future requirements.” What Source3 promises is to let designers everywhere simply upload a 3D model file and a texture to be rendered for online visualisation. While that is happening, the designer accesses a screen that lets him or her set the title, insert all metadata information, select categories and add tag words. Once this phase is complete, all the designer has to do is select the marketplaces and e-tailers that s/he wants his or her product to be featured in and Source3 will do all the work. Personally, I know quite a few people that could already take advantage of this service, instead of manually uploading to Shapeways, i-materialise, 3DaGoGo, ToyFabb, Pinshape, Cults, Threeding, Shapetizer… “Once a month, a revenue report comes from each of these marketplaces. We look at it and distribute the money earned to the designers based on how well their products sold. Give us your Pay{al account and we’ll deposit the money, and if you want to see a detailed report, we can provide the number of products sold, where the buyers are located, etc.,” Tom explains. “So, we’re slowly opening up this ecosystem of distribution marketplaces… The next step is to get beyond that and get closer to the more traditional marketplaces, like Amazon, or Etsy, as what we’re doing on the backend as well is forming relationships with 3D printing partners like White Clouds. If we set up for example your ability to distribute at Etsy, once the product is purchased it would send a a request to White Clouds to get it manufactured on demand.” The bottom line is that Source3 is being created to enable designers to just focus on designing and not worry about distribution. The real “unicorn” that Source3 is chasing is a “derivative rights model”, working with top brands to create consumer products out of their IPs and distributing through all the 3D printing marketplaces that have been integrated into the network. As in the recent Capcom and Zverse collaboration for the Street Fighter videogame cover art, or the following one with CDbaby. In order to achieve this, Source3 combined the experiences of two different “souls”. Some of the founders come from a digital rights management company called RightsFlow, which was created to manage royalties and licenses for digitally distributed music content. They were there when Sean Parker presented the Napster project to Universal Music in 2001 and the company was later acquired by YouTube. Another part of Source3 comes from Geomagic, which was acquired by 3D Systems and saw what happened with the home 3D printing hype. With physical objects, everything is much more complex but there are services like 3D Hubs which, as Tom puts it, “are already very functional processes.” Others, like Autodesk Spark (which is one of Source3’s investors) are working to create the ecosystem for global digital manufacturing. This might not mean that giants of the toy or consumer product industry will see it as a threat but some of the smaller companies might. The digital manufacturing revolution is happening, preparing to manage it can only be beneficial for everyone. If I were a designer of 3D printable products, I would definitely give the beta a shot. Despite posing certain potential threats to brands and intellectual property, 3D printing also offers a wealth of benefits, including customization for the customer and zero inventory costs for the manufacturer, making 3D printing a potential win-win means of manufacturing. Kudos to Viacom and South Park Studios for embracing 3D technology via licensing and setting an example in the industry for other brands determining the best way to react to this promising, disruptive technology.
















































































































































































































































































































































































































































































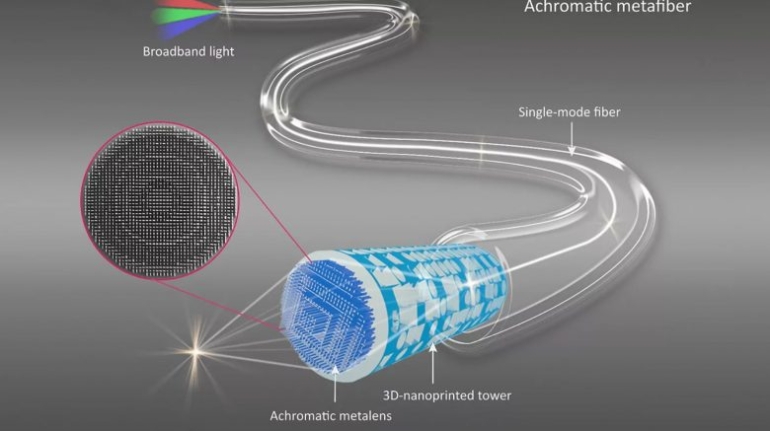
















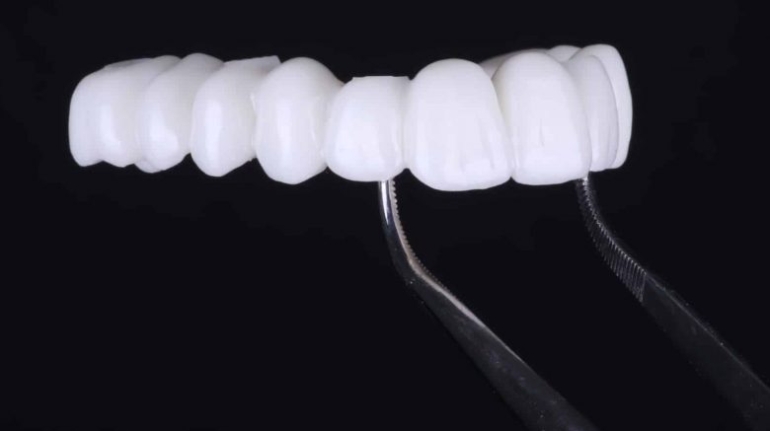

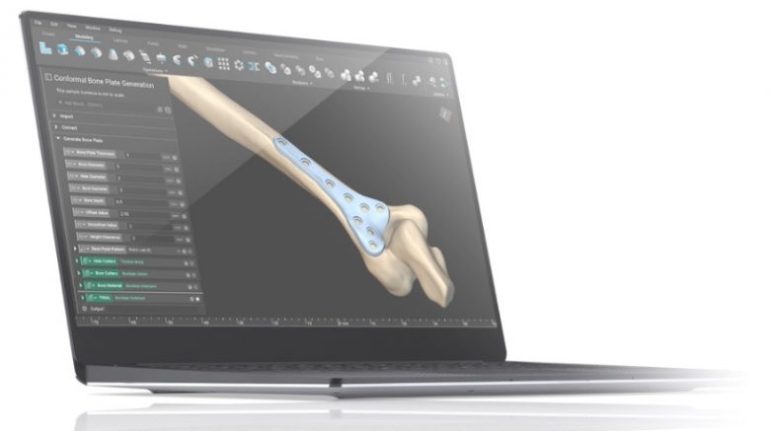













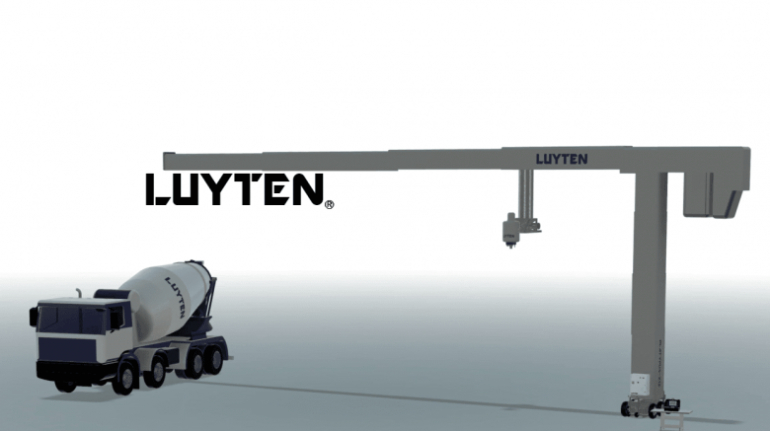

























































































































































































































































































































































































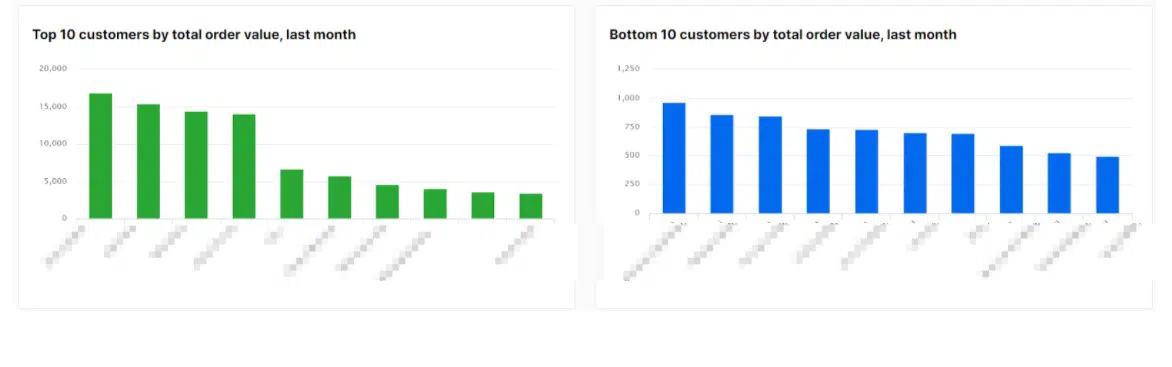




































































































































































































































































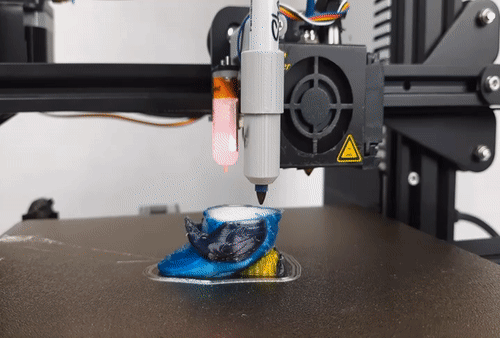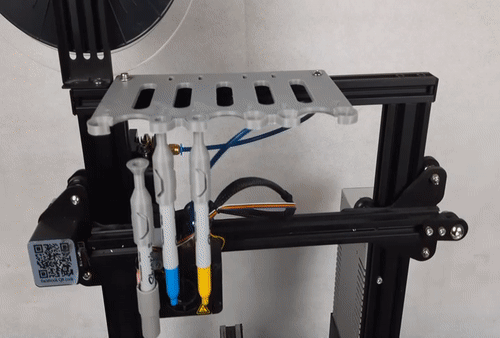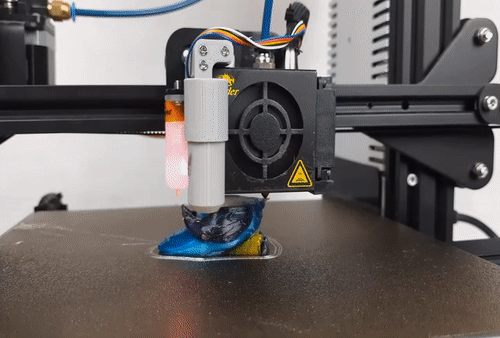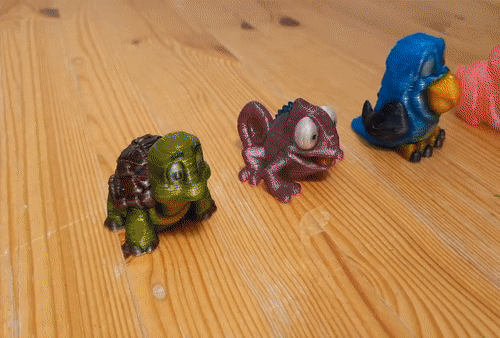








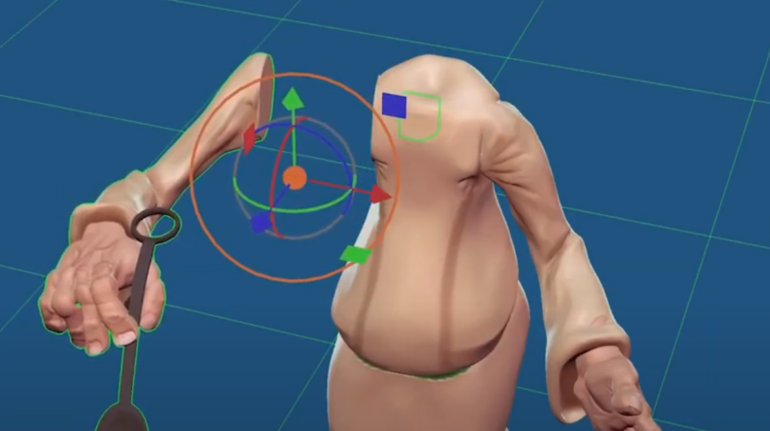

















































































































































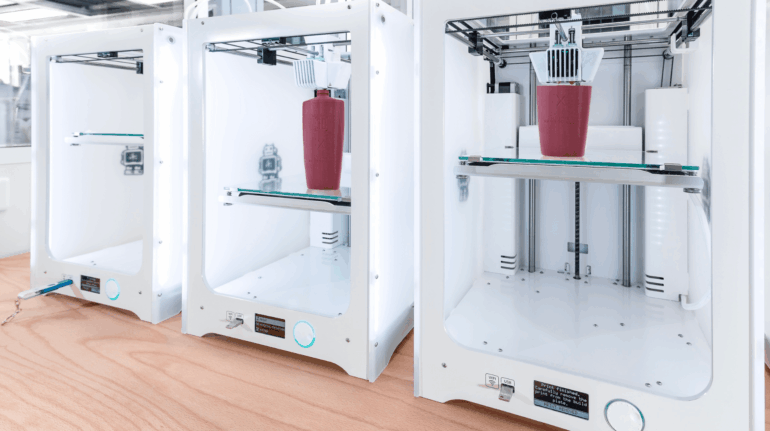









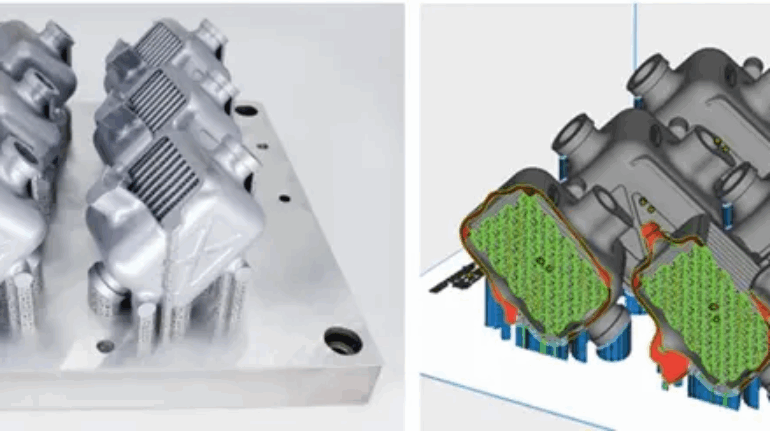
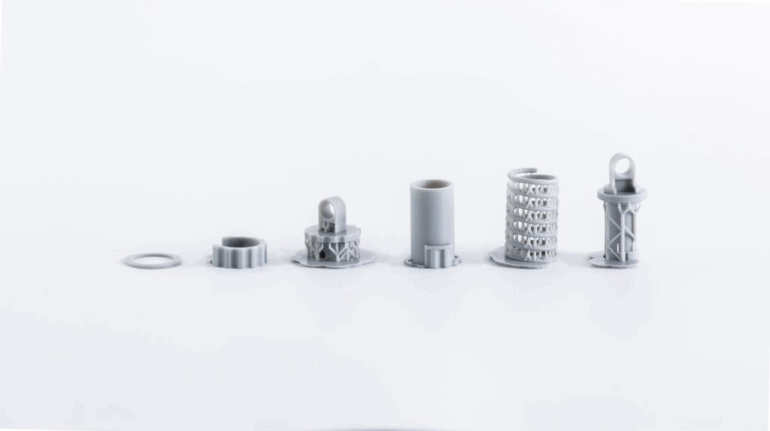










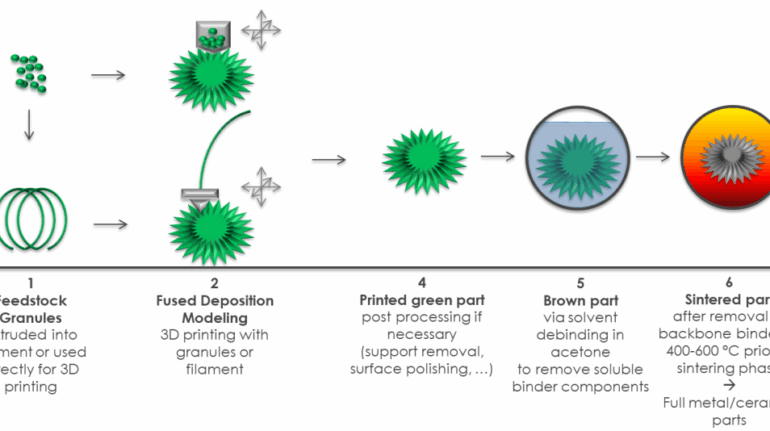
























































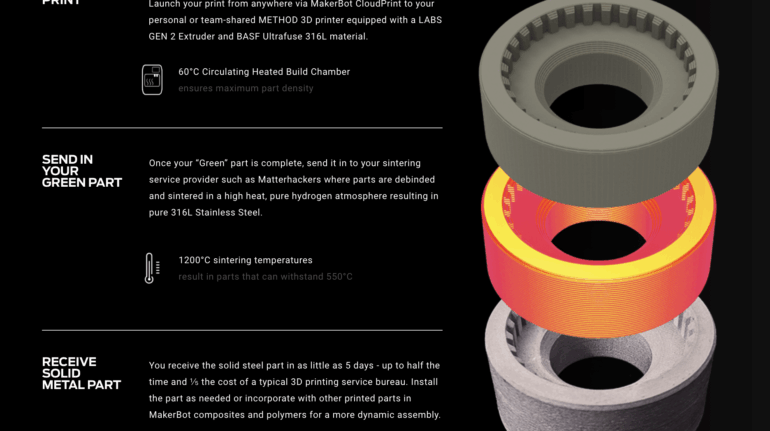



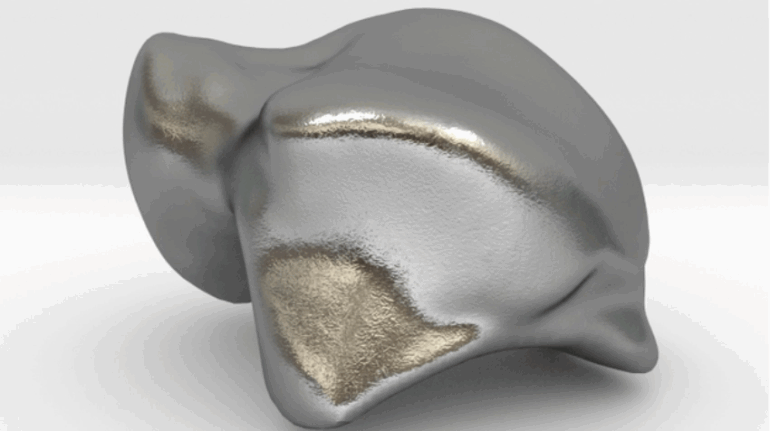






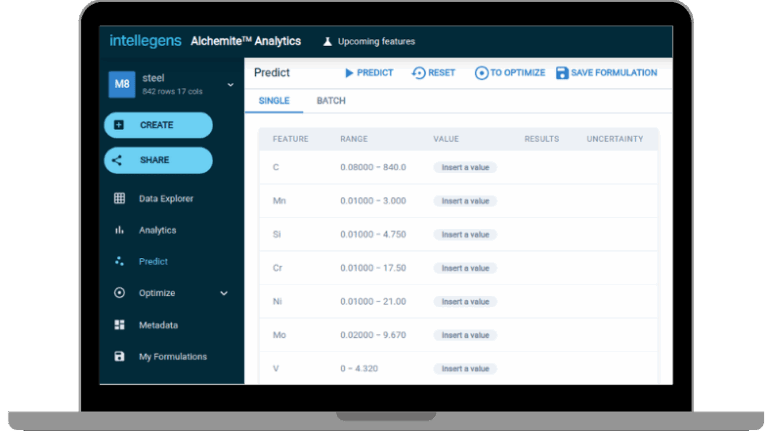





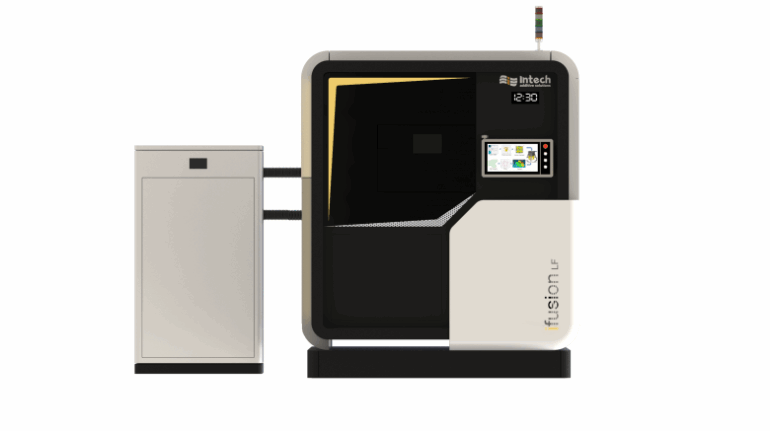


















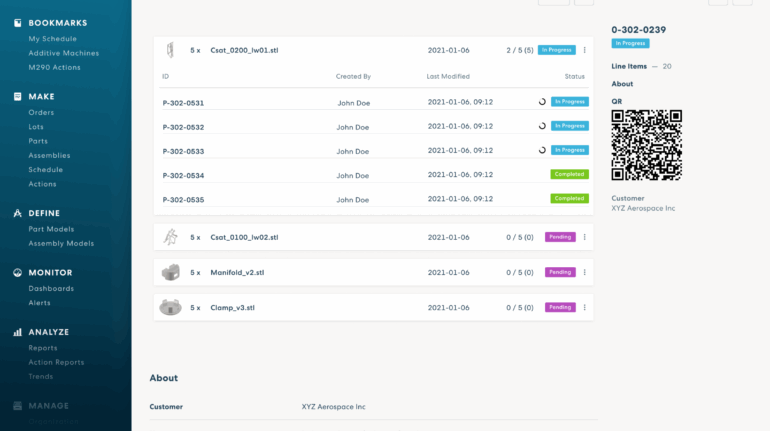

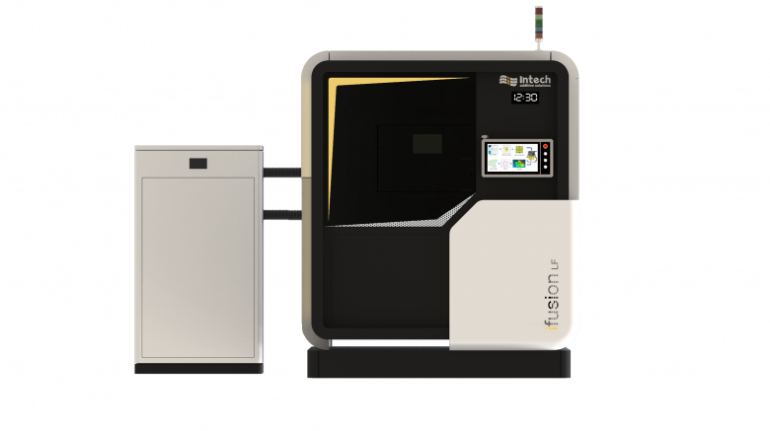

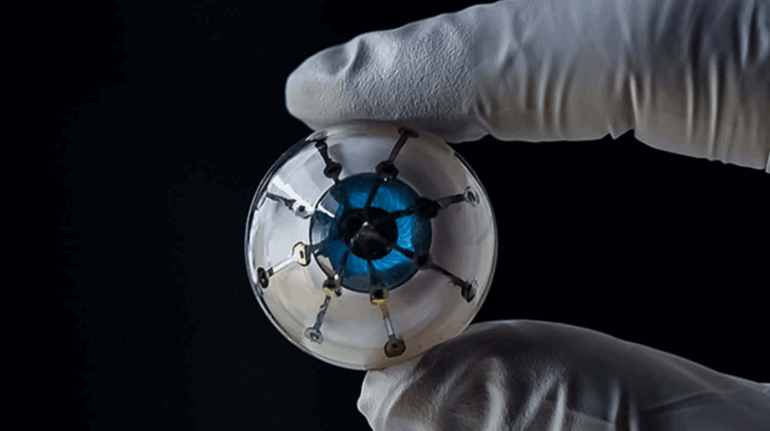
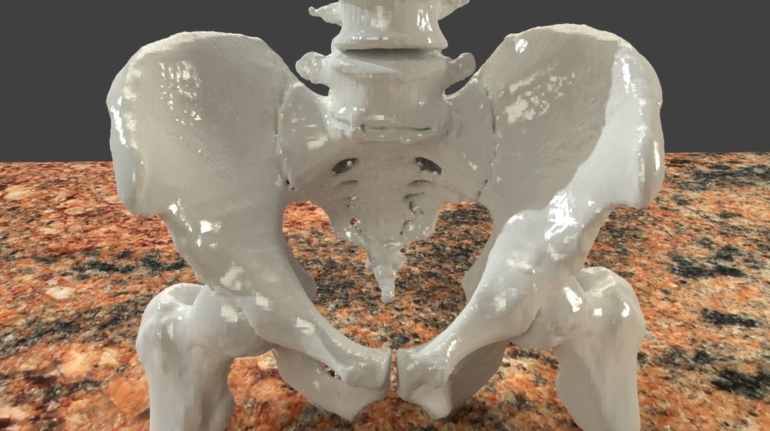







































































































































































































































































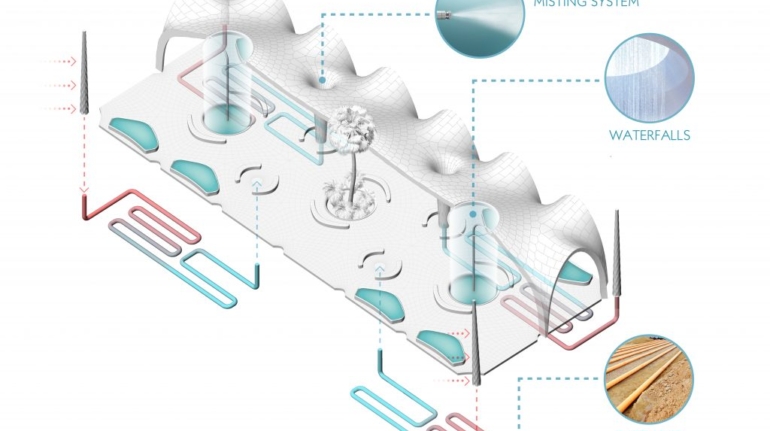



















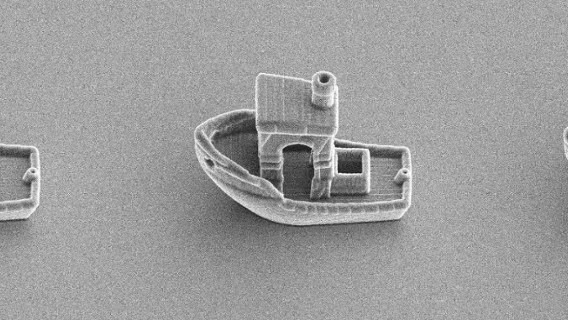

























































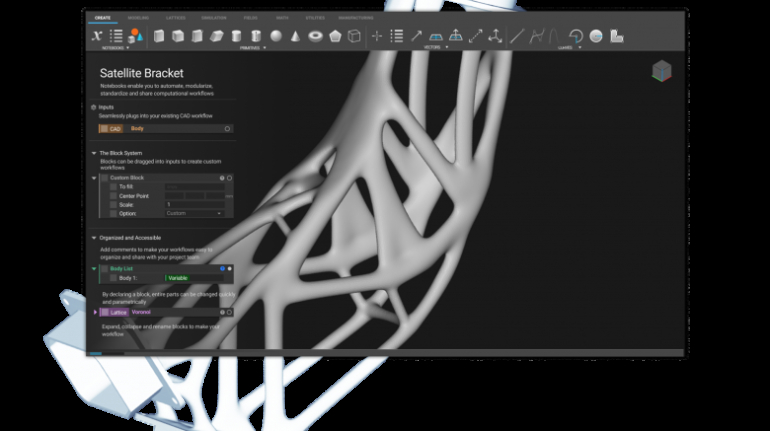



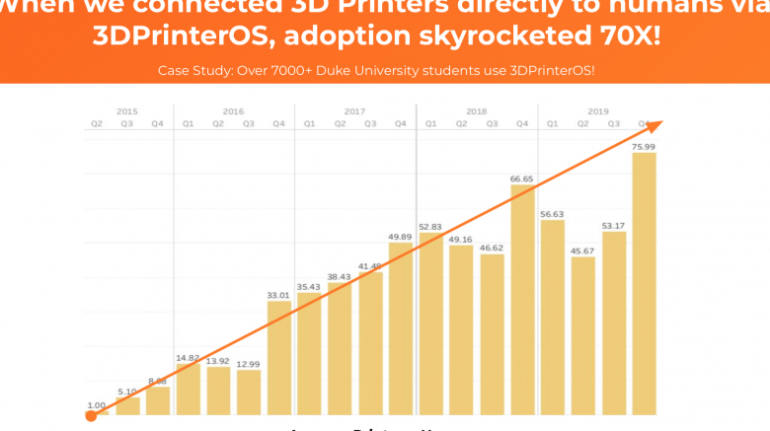



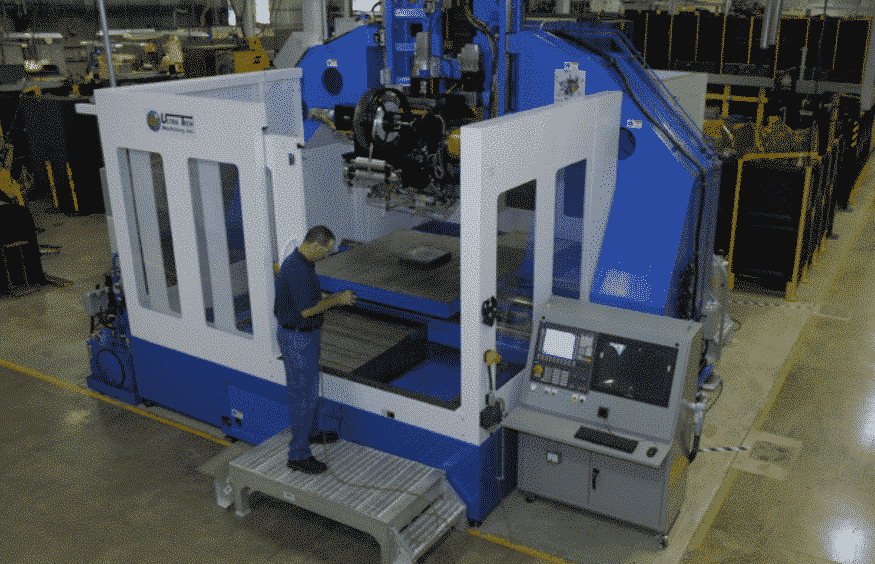









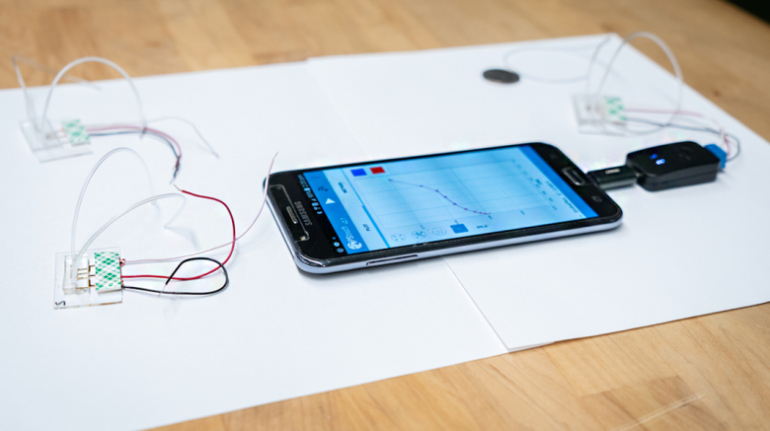


























































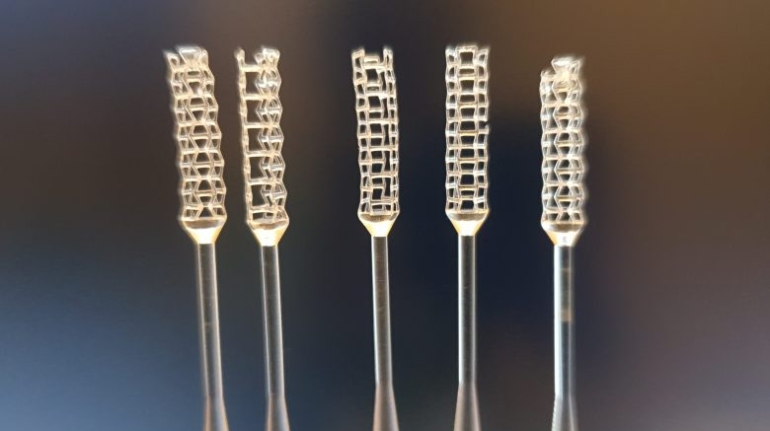
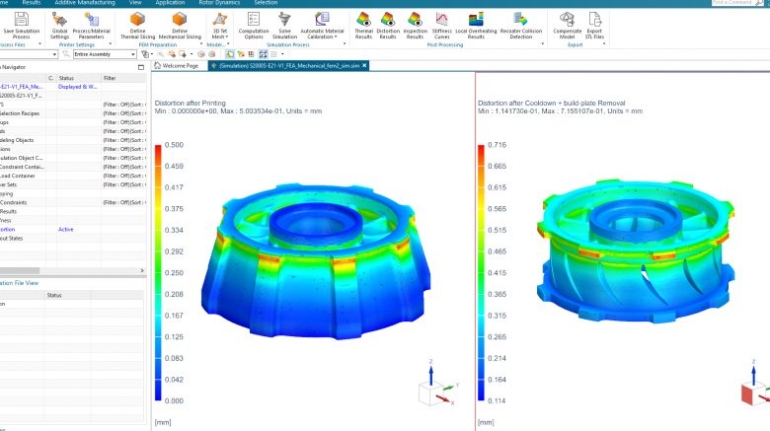

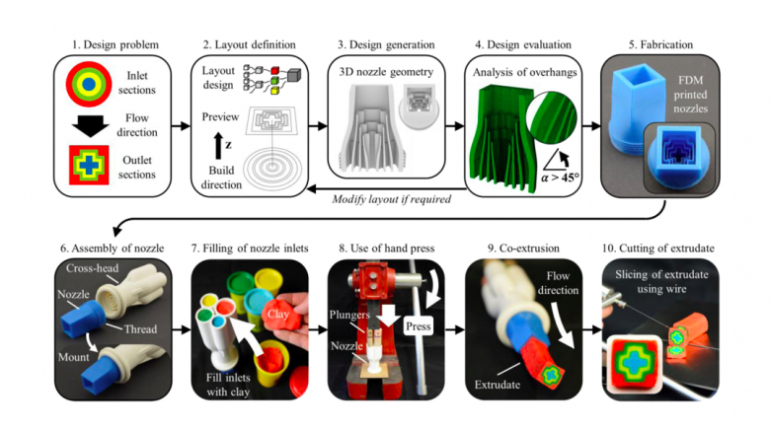
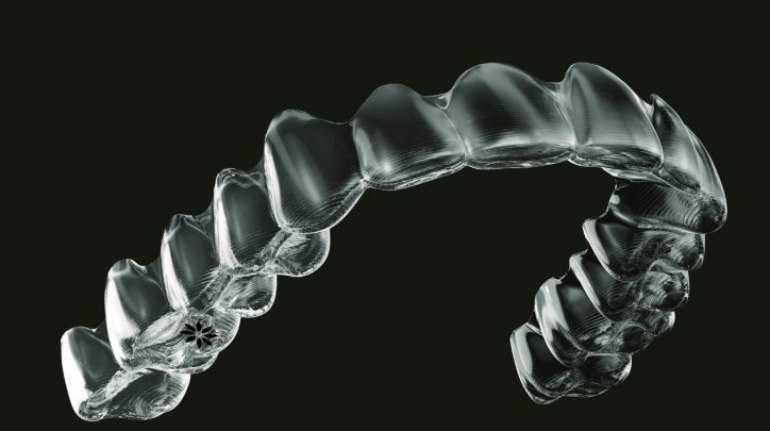


















































































































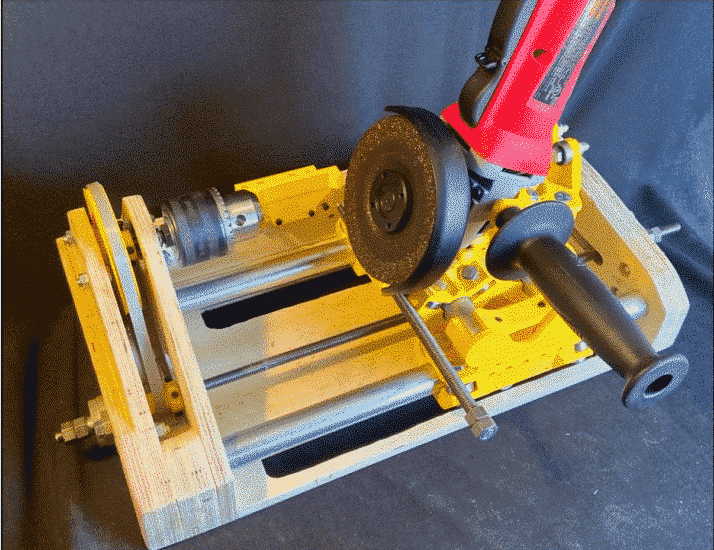



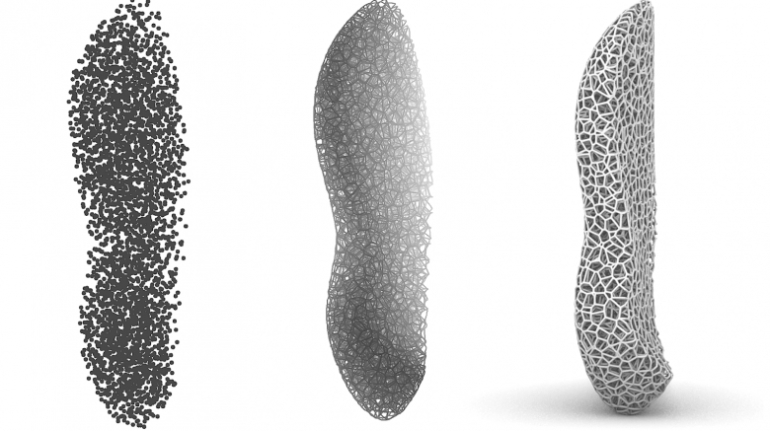

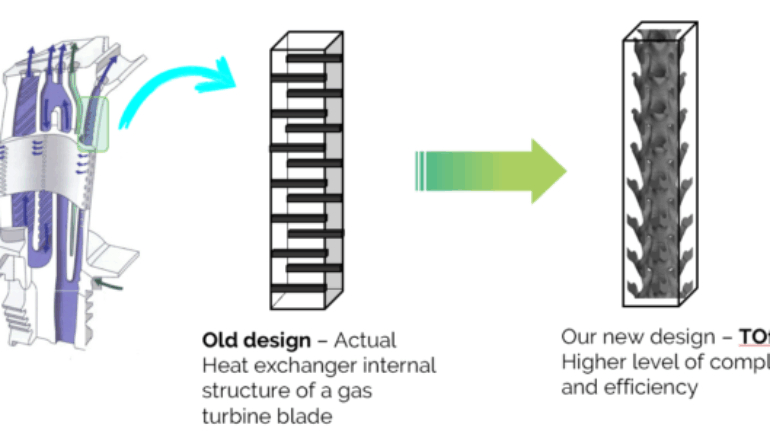








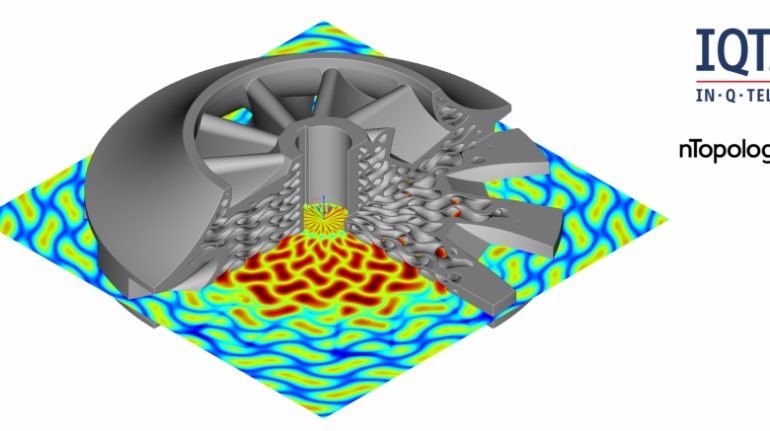
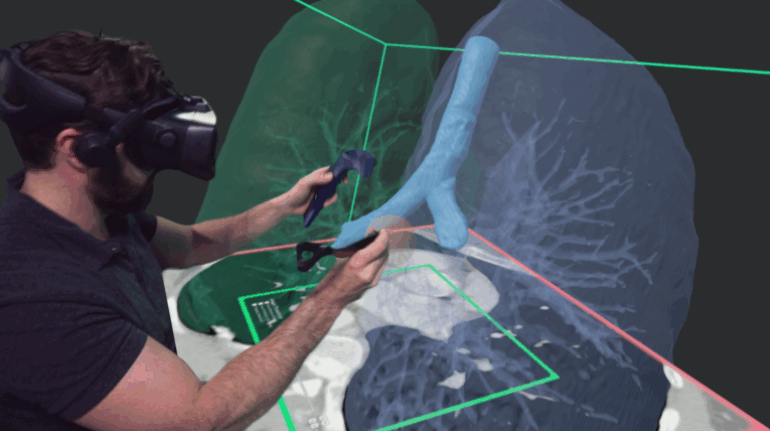










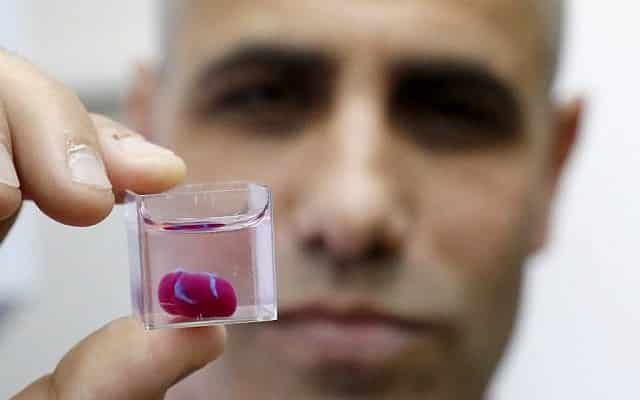







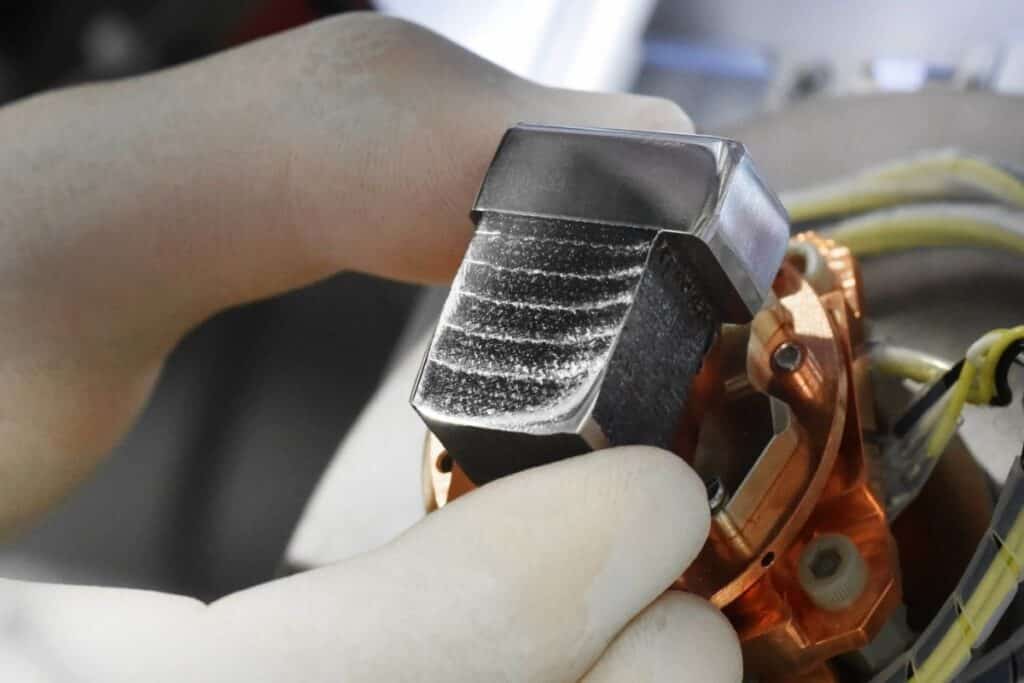
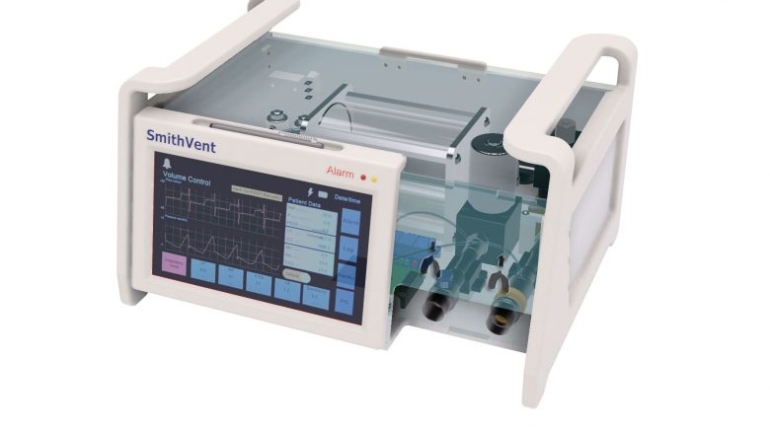
























































































































































































![[GIVEAWAY] Animal Crossing and More Accessories You Could 3D Print for Your Nintendo Switch [GIVEAWAY] Animal Crossing and More Accessories You Could 3D Print for Your Nintendo Switch](https://facfox.com/wp-content/uploads/2020/04/Untitled-9.png)














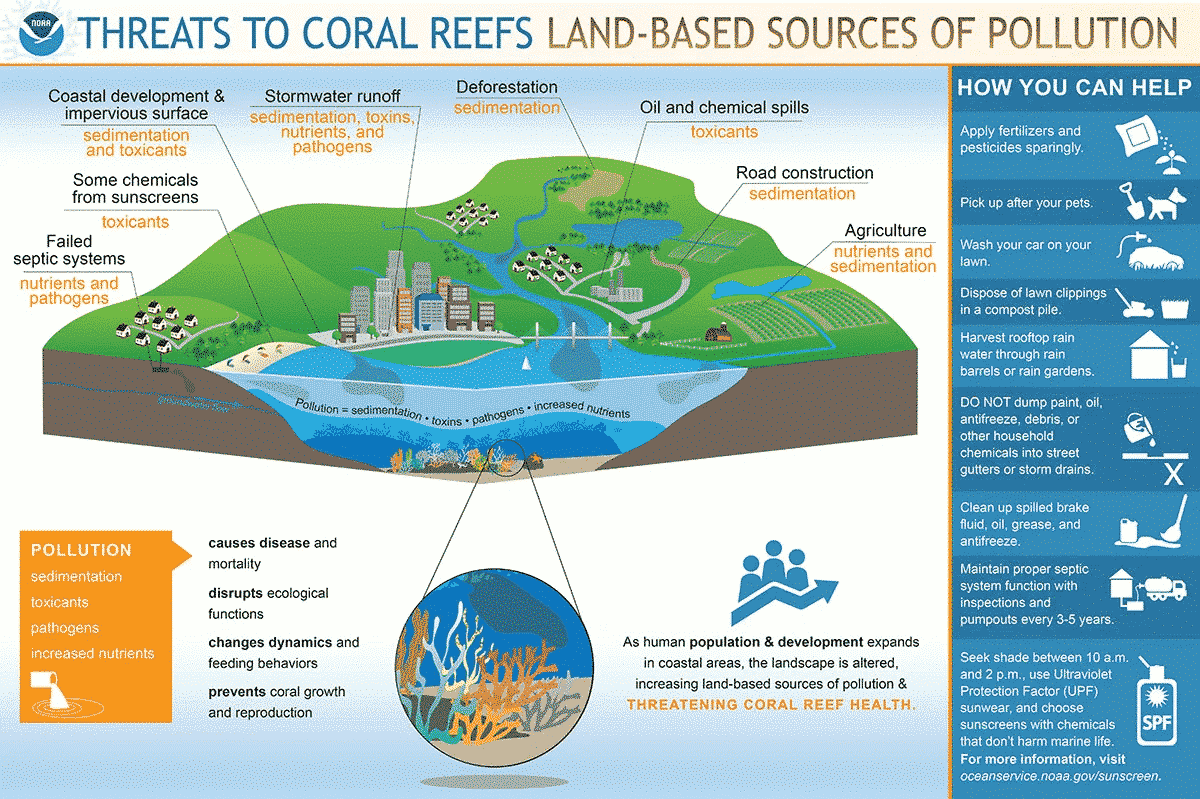









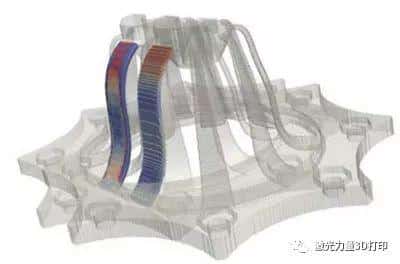
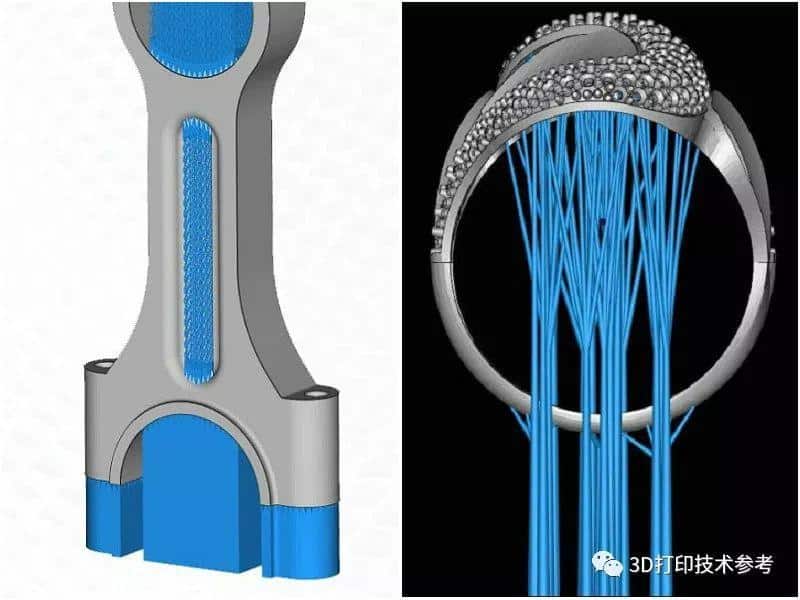

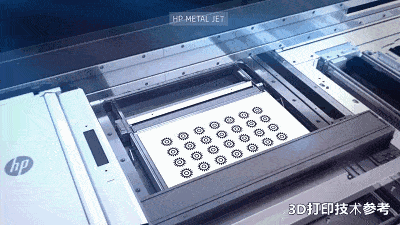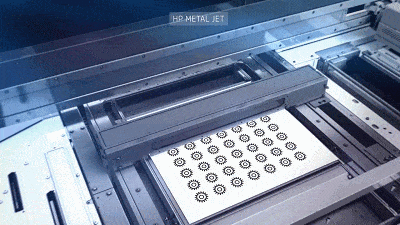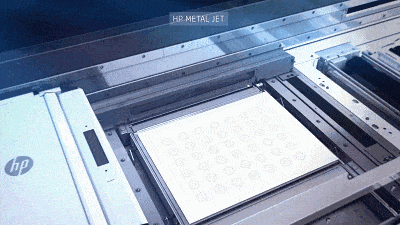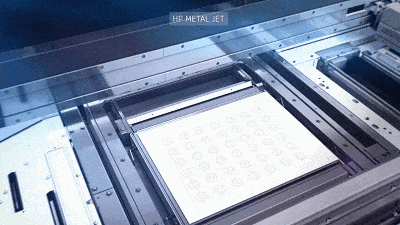
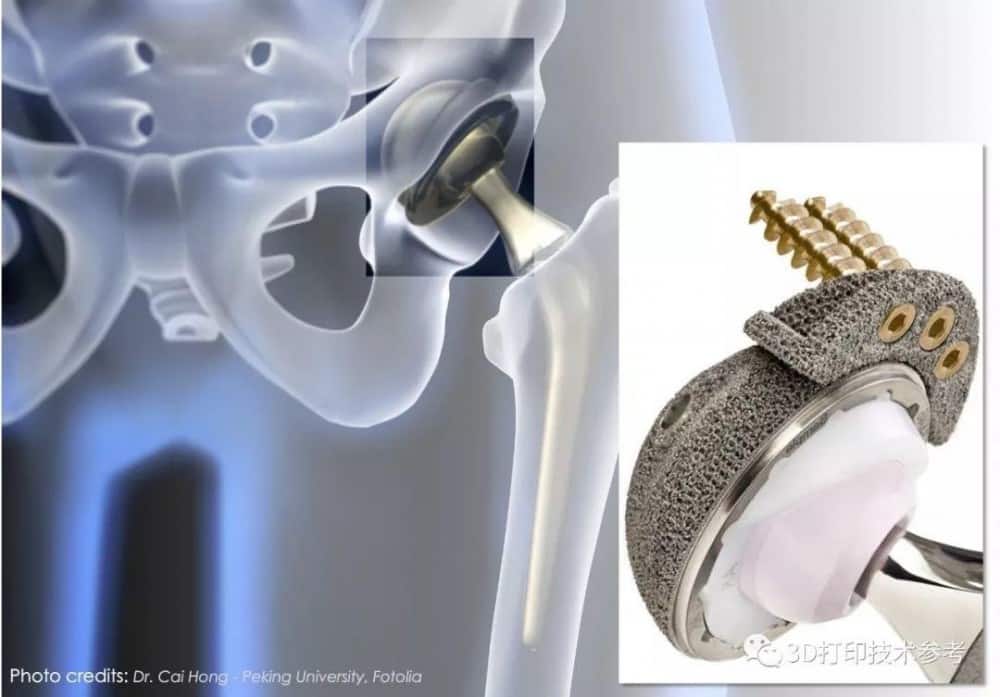
![[Additive Manufacturing] Get the standard out [Additive Manufacturing] Get the standard out](https://facfox.com/wp-content/uploads/2020/04/20200408_5e8ddb8435cbe-1.jpg)
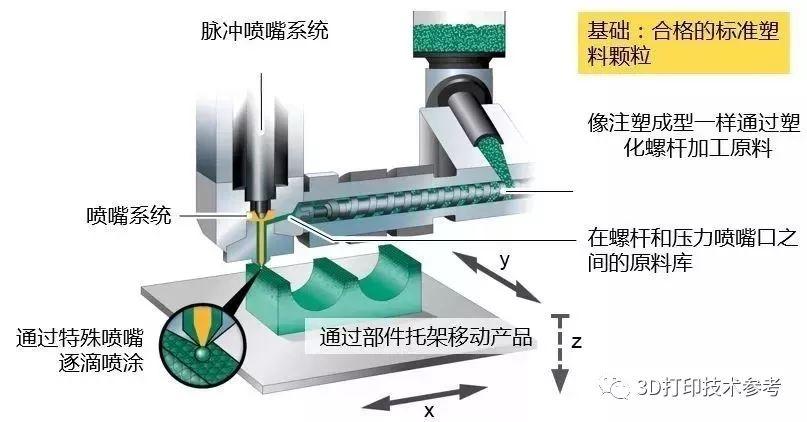








































































































































































































































































































































































































































































































































![SPEE3D shows of new, larger WarpSPEE3D cold spray AM system [video] 3D Printing Processes SPEE3D shows of new, larger WarpSPEE3D cold spray AM system [video] 3D Printing Processes](https://facfox.com/wp-content/uploads/orderfile/image/2020/11/yYvuea.jpg)


































































































































































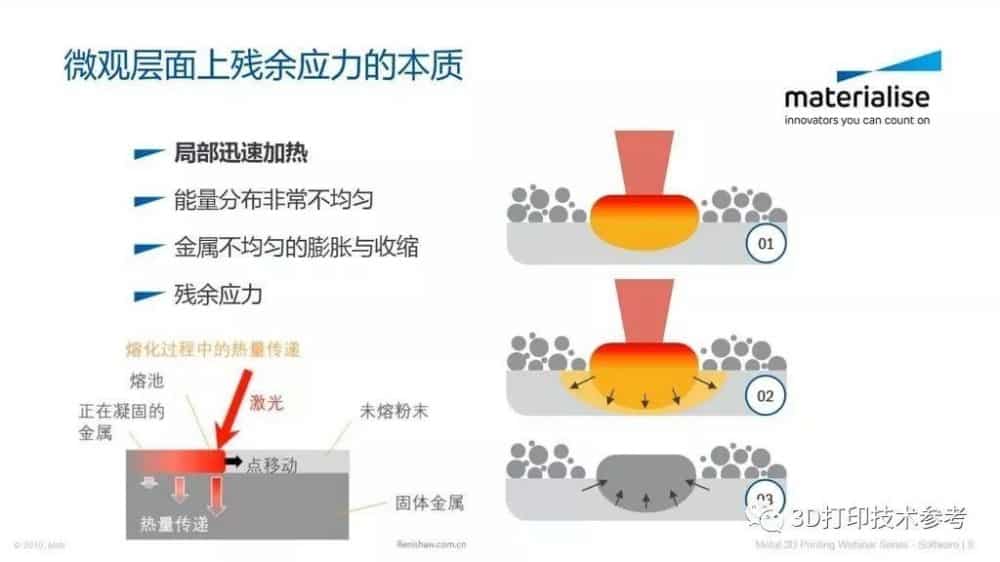
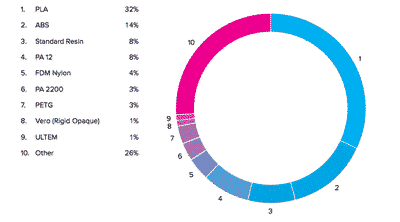



























































































































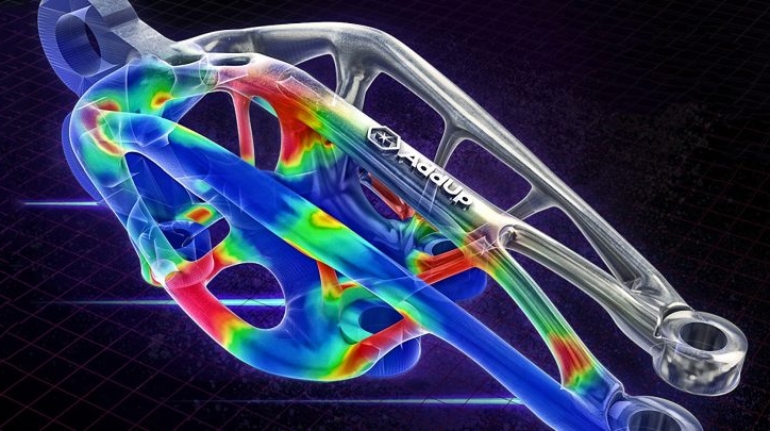





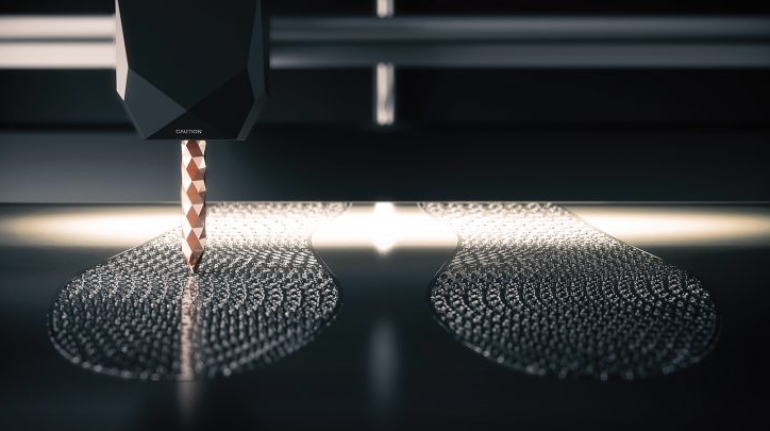




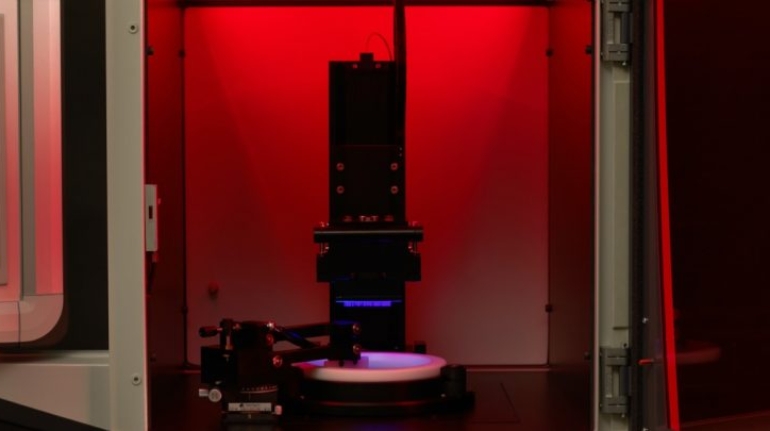






























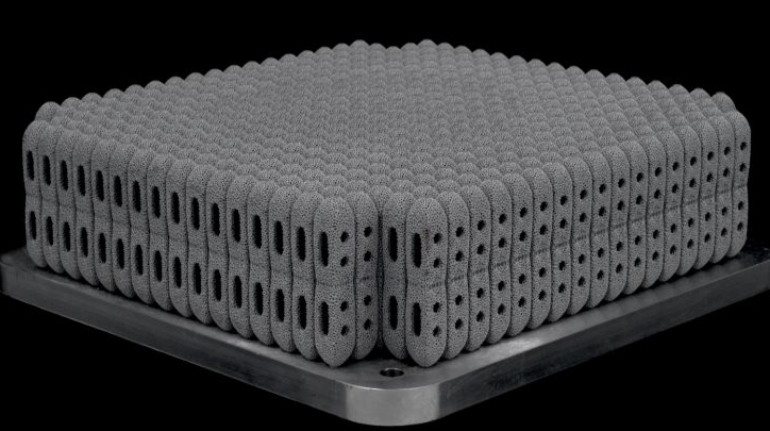






























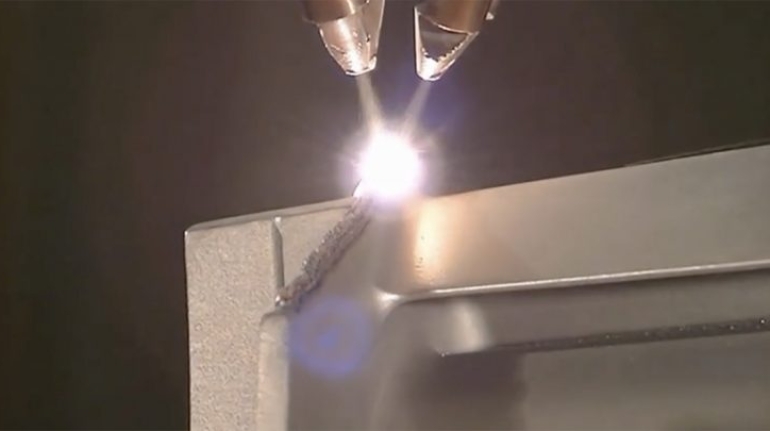


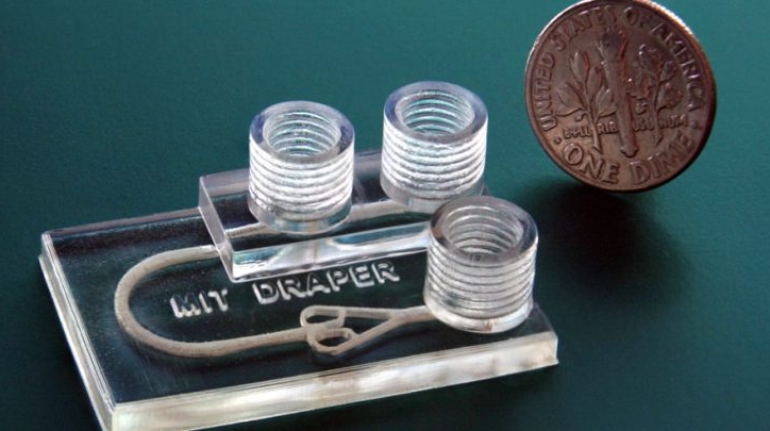


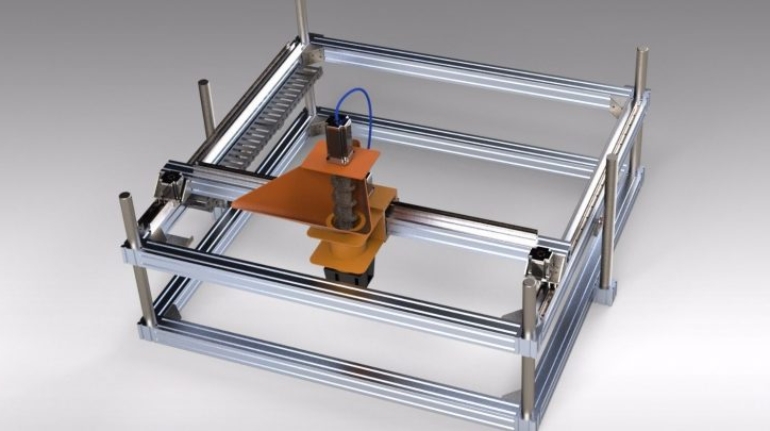



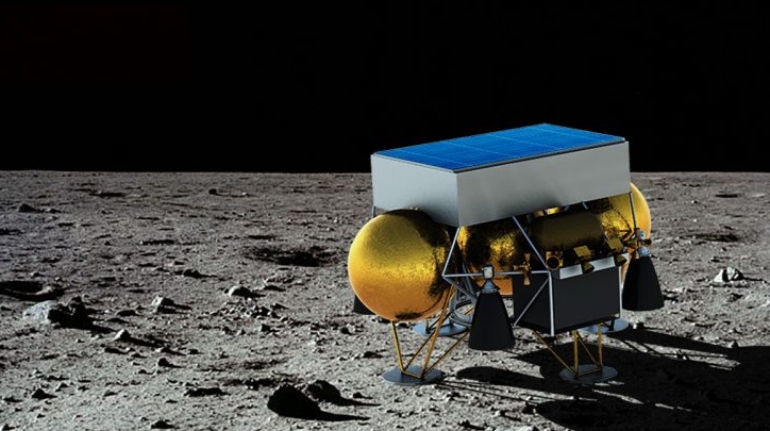










































































































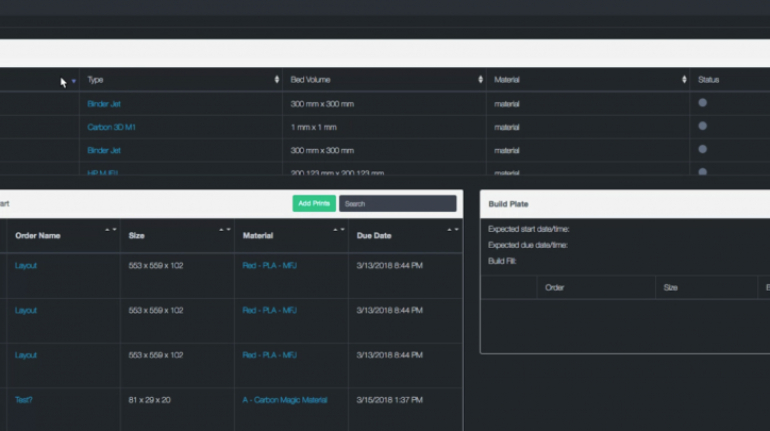


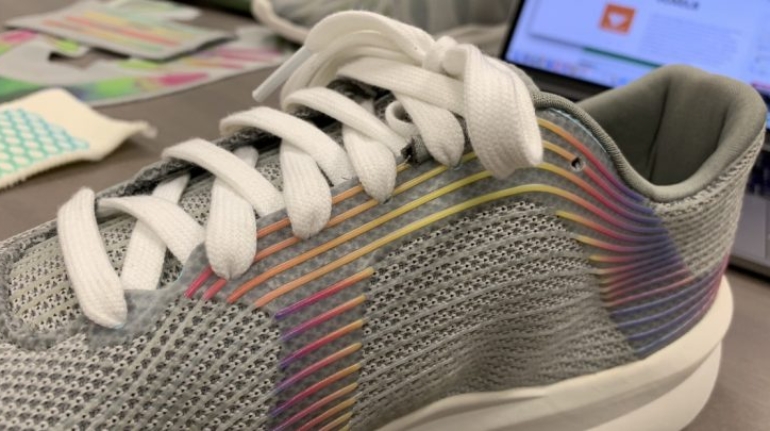


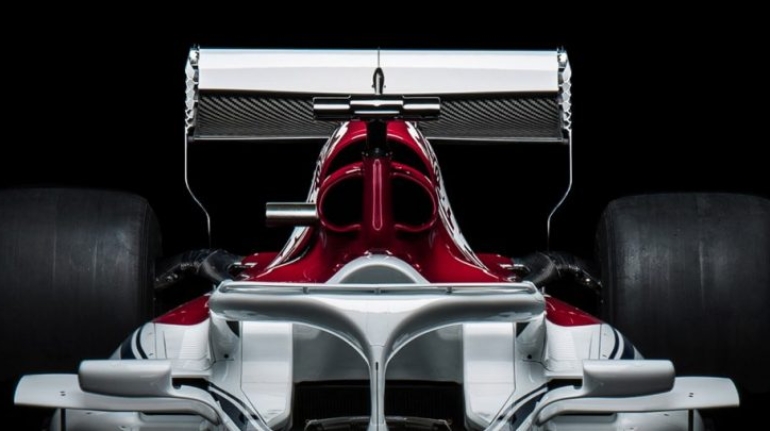



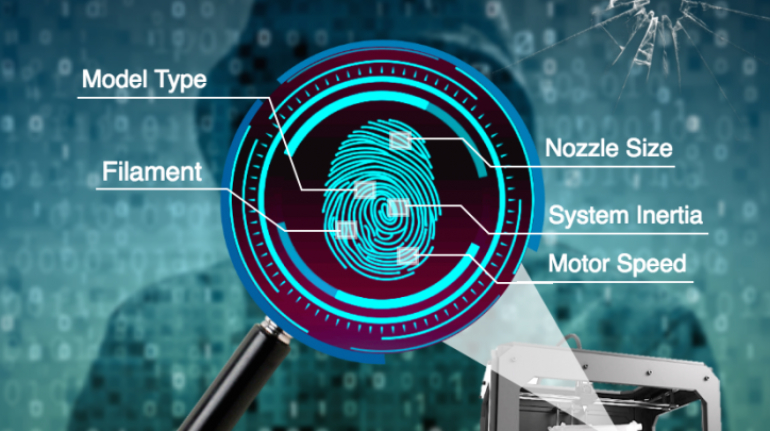












































































































































































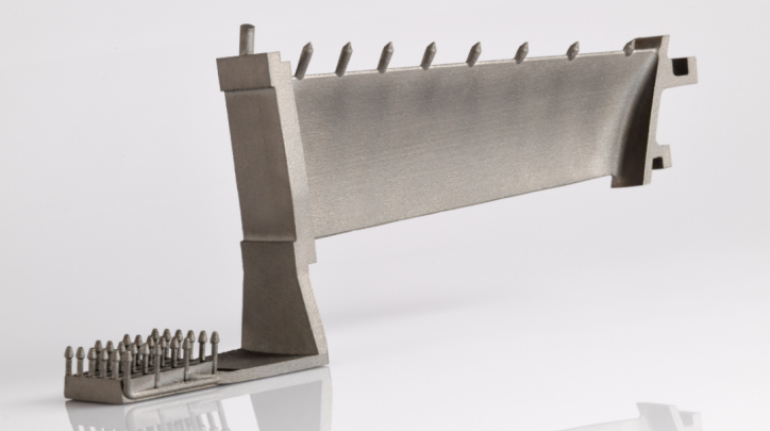



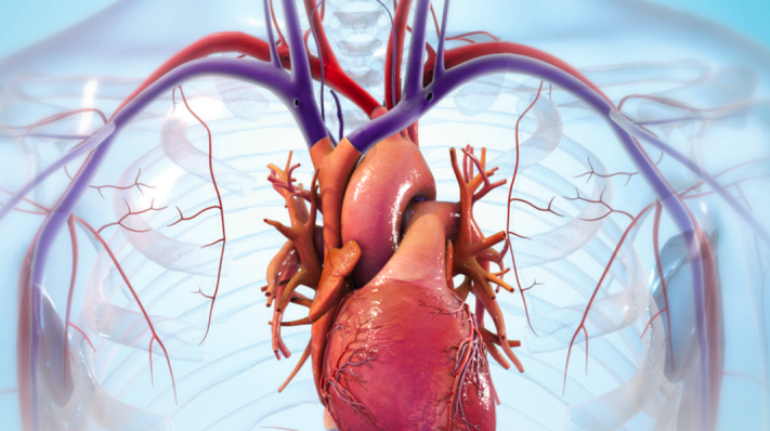

















































































































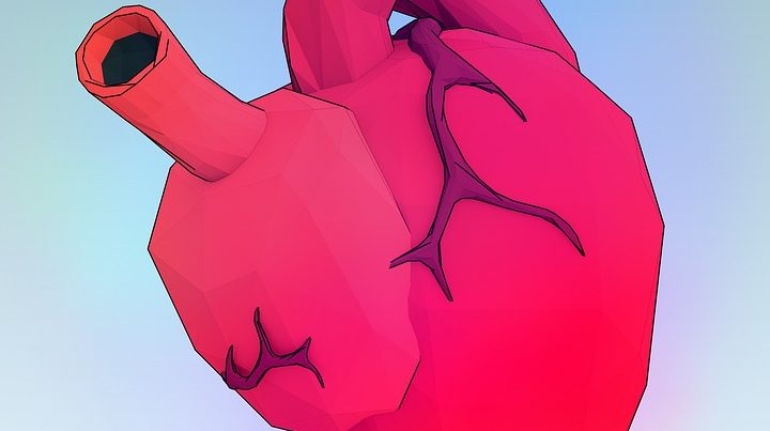


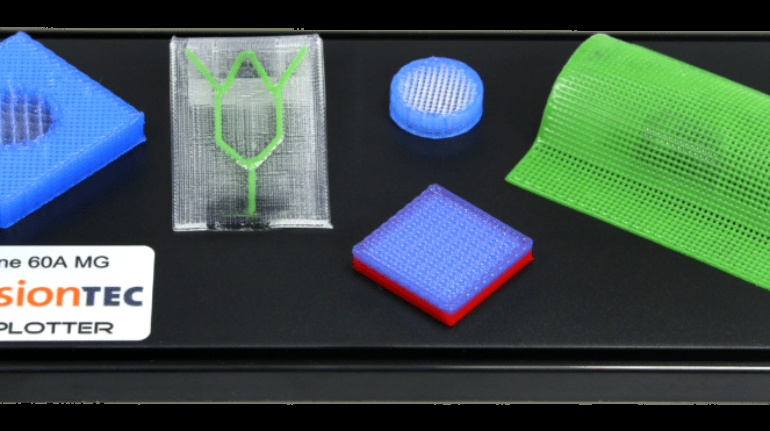











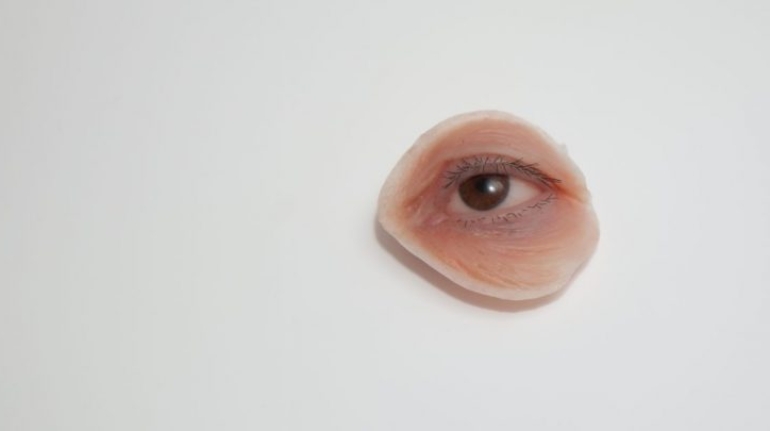


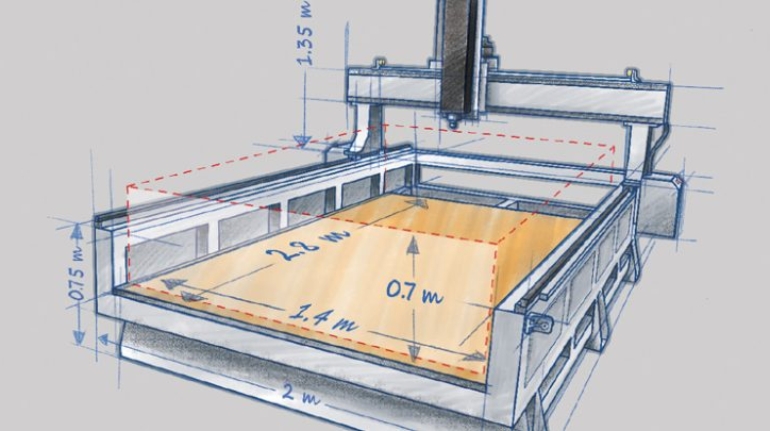





























































































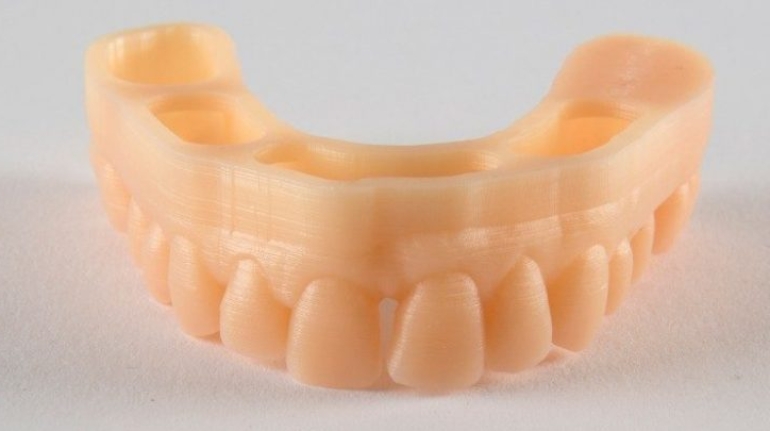
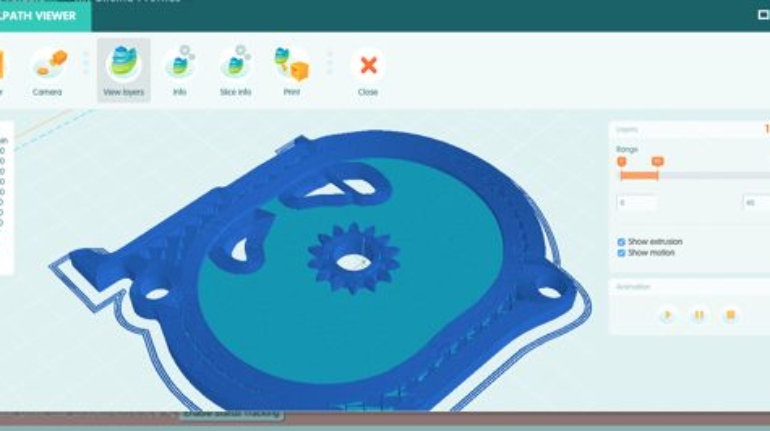
![Mosaic releases new chroma 3 software for palette+ multi-color FDM [Video] Marketing and Content Mosaic releases new chroma 3 software for palette+ multi-color FDM [Video] Marketing and Content](https://facfox.com/wp-content/uploads/orderfile/image/2020/11/na2u6f.jpg)


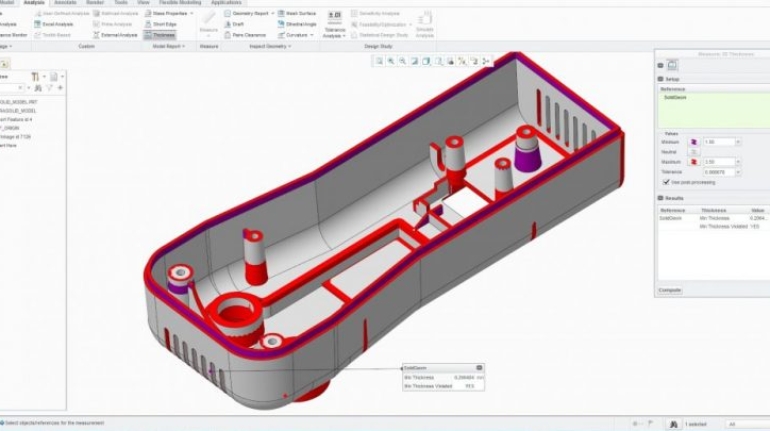












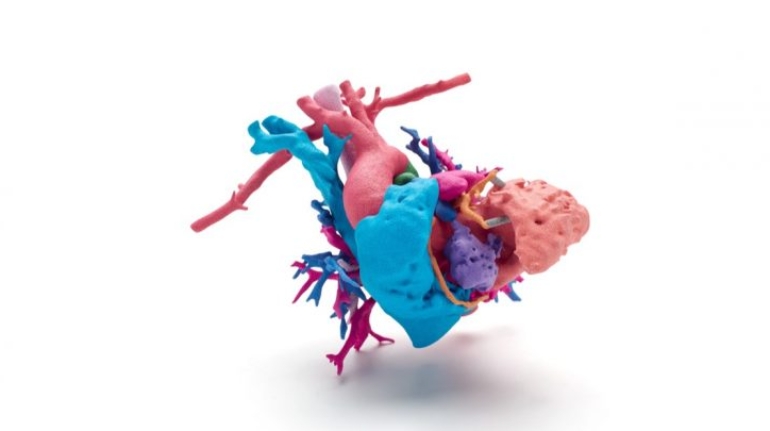















































































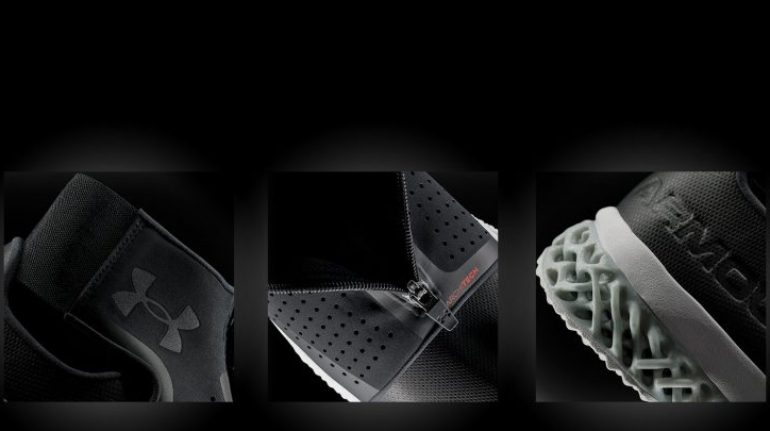











































































































































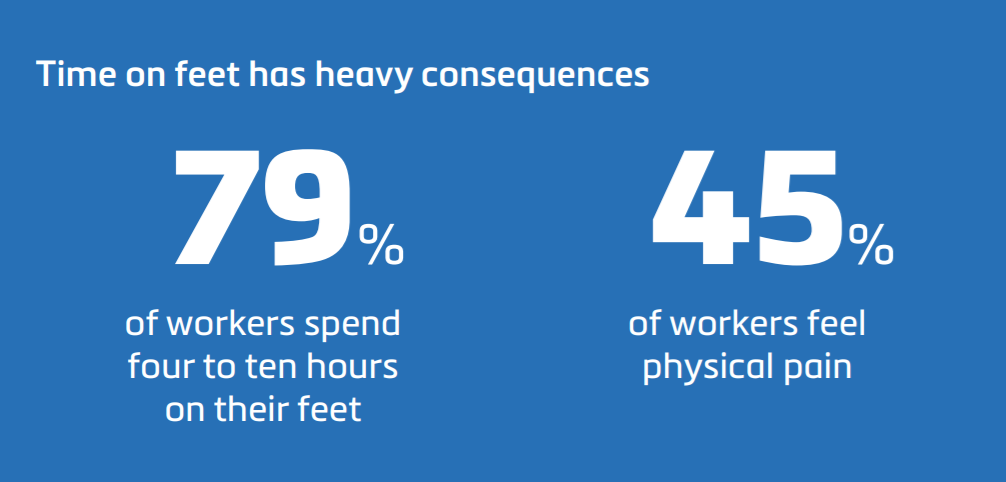
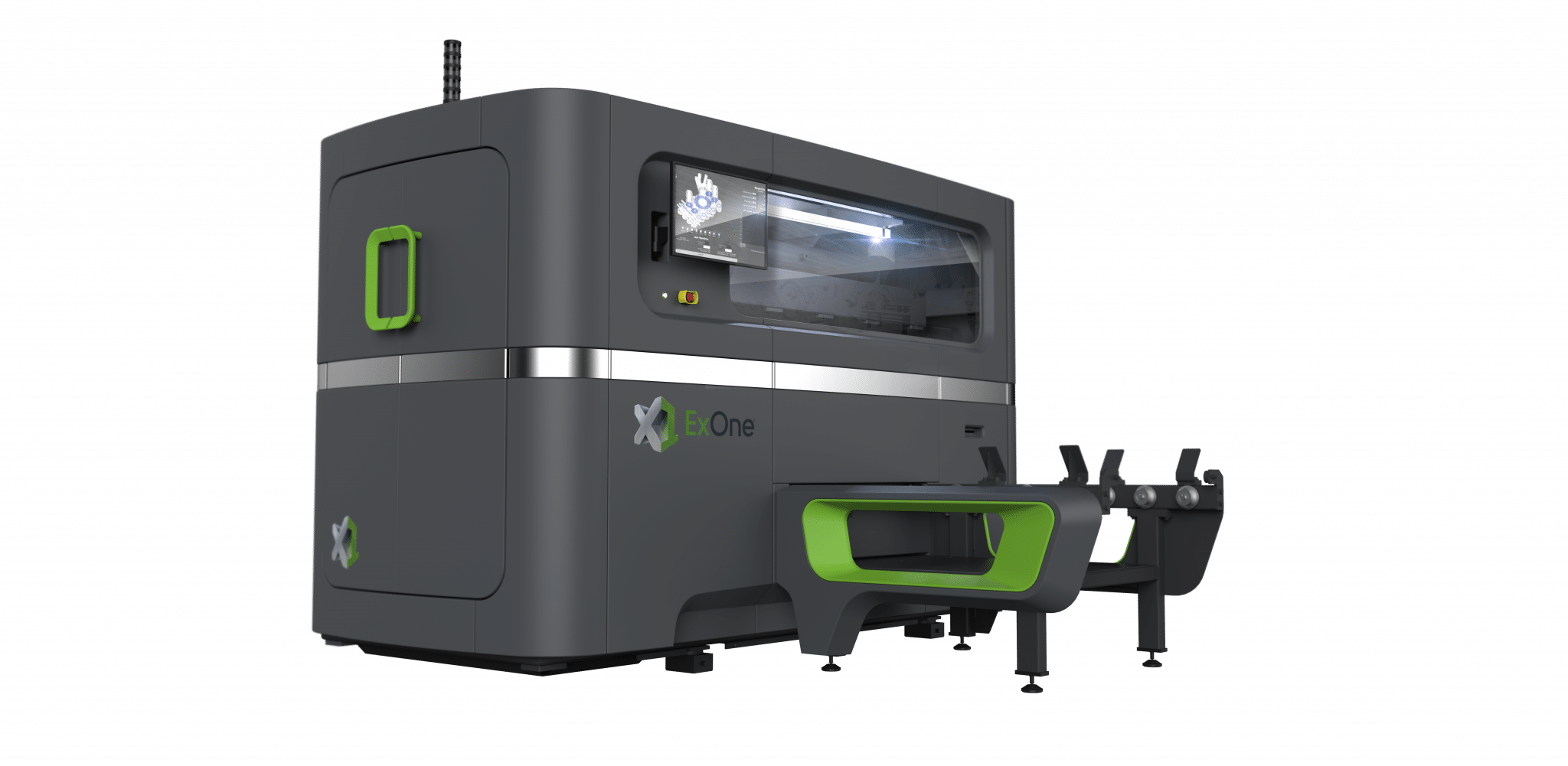
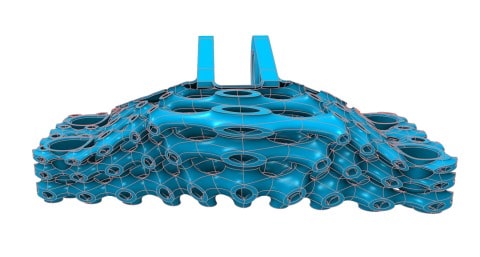
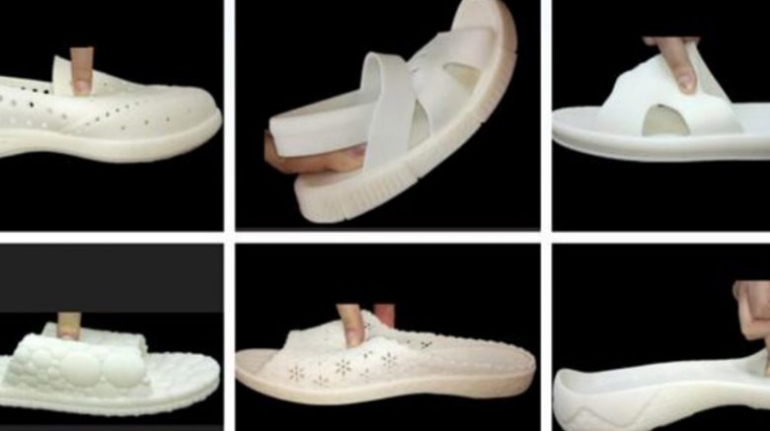
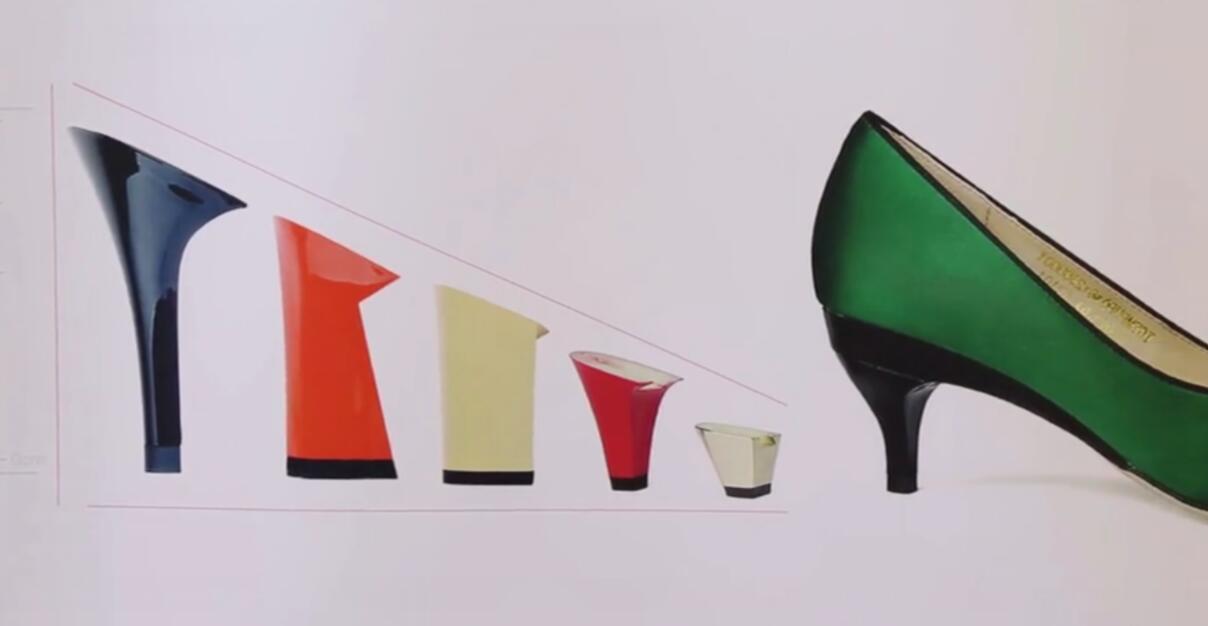
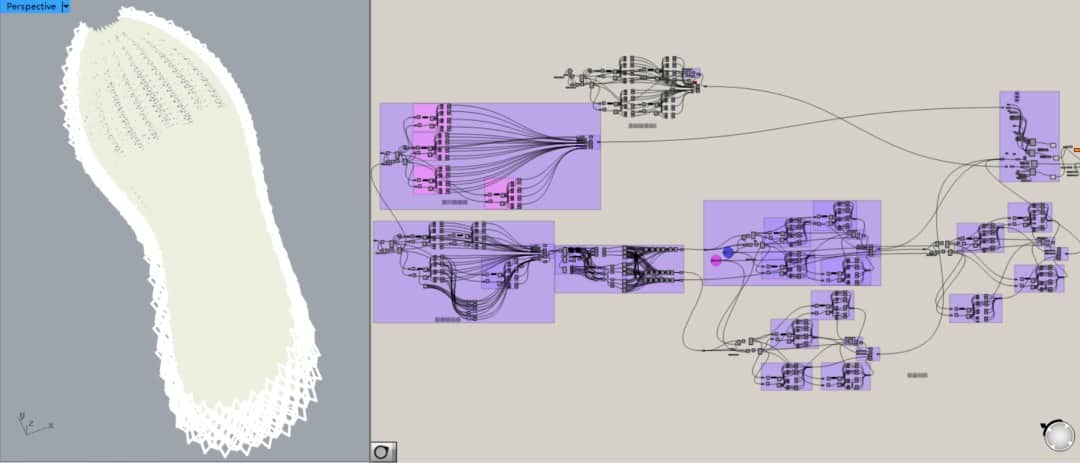
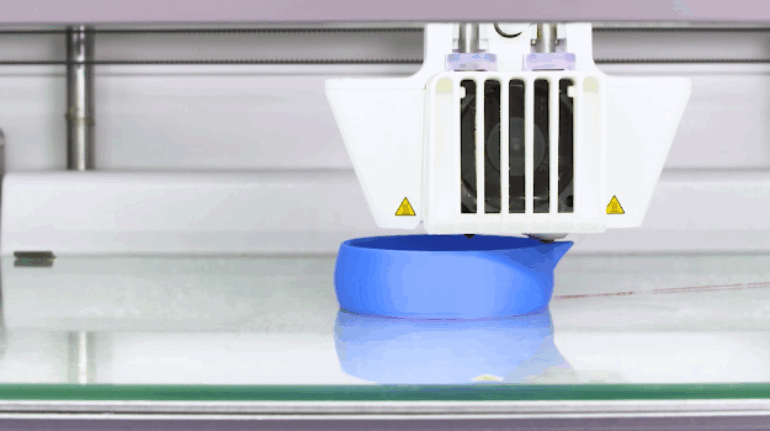
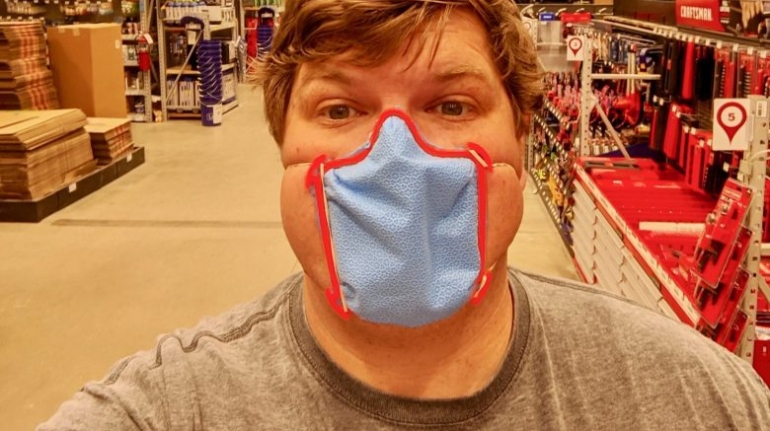
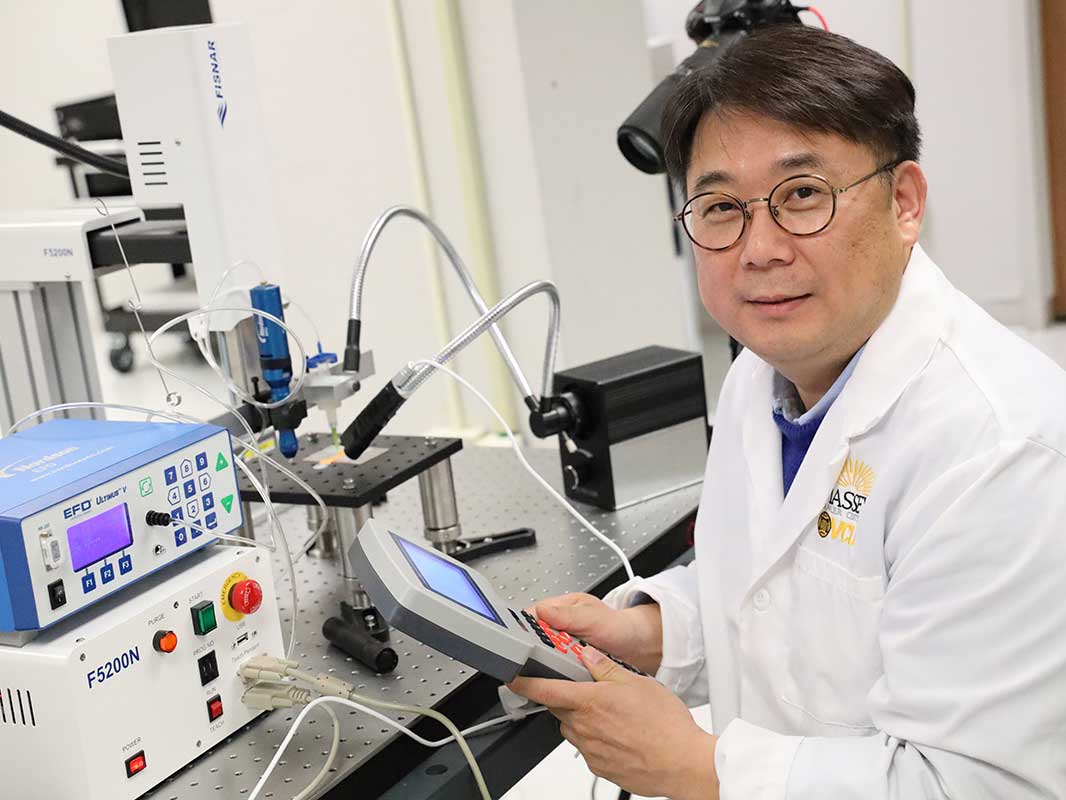
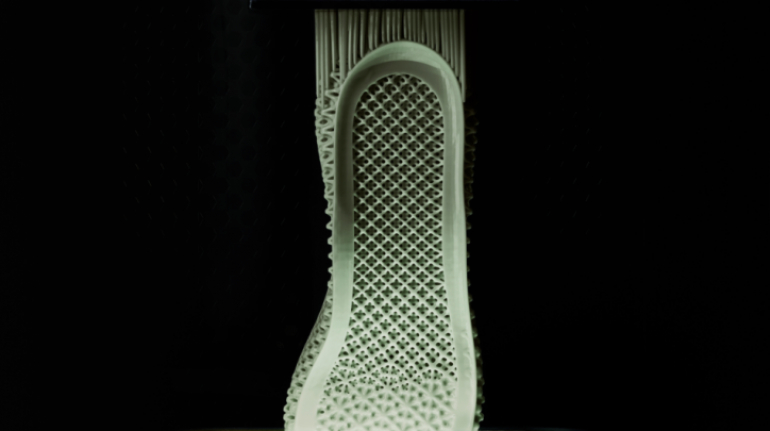
![EnvisionTEC launches new E-Shore A Material for 3D printed footwear [video] Consumer Products EnvisionTEC launches new E-Shore A Material for 3D printed footwear [video] Consumer Products](https://facfox.com/wp-content/uploads/orderfile/image/2020/11/ERrIja.jpg)