Munich-based chemical firm Linde Group and clinical printing specialist 3D Medlab have announced the results of testing their new process gas to optimize the 3D printing of medical components.
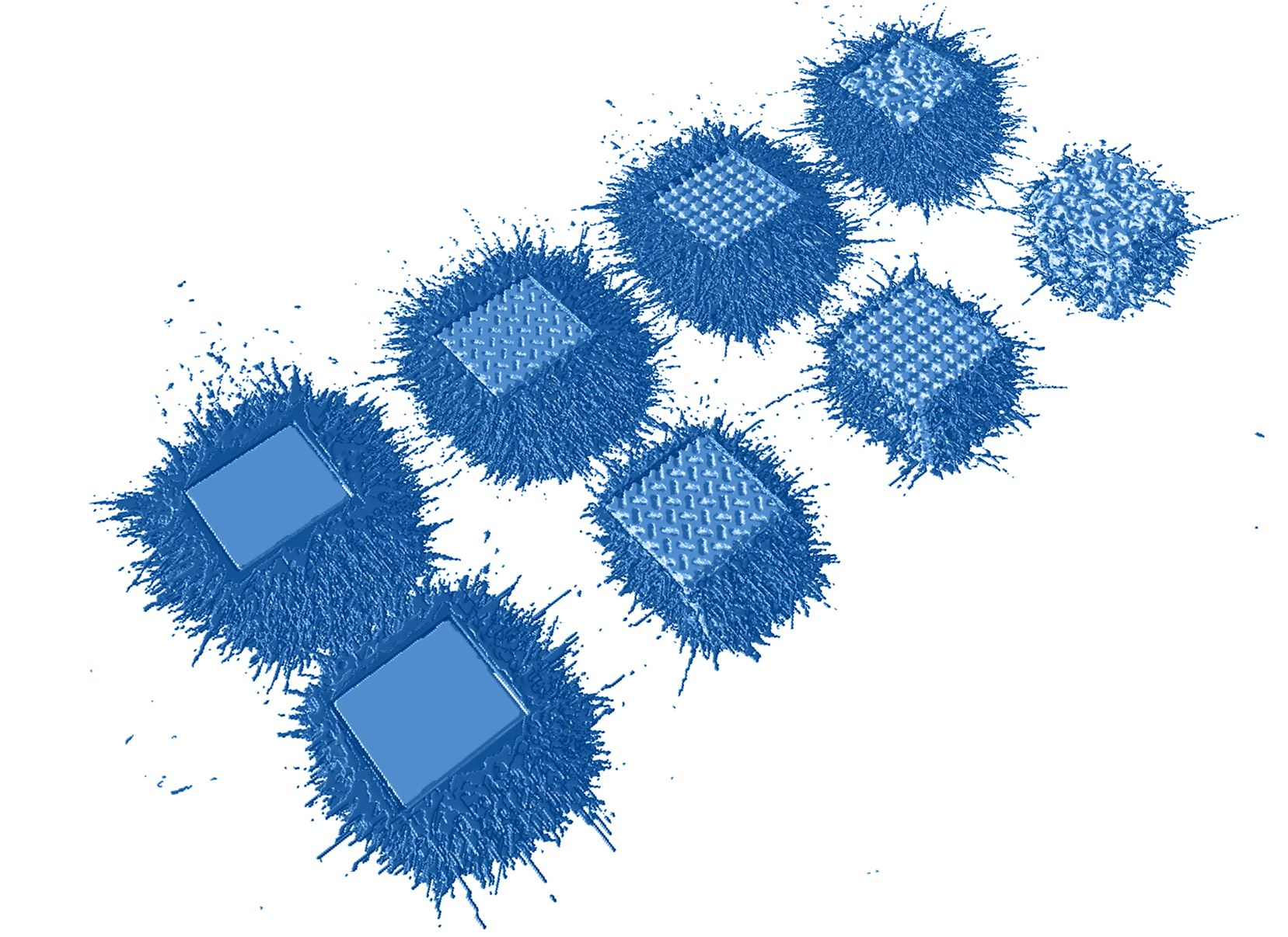
The study, which took place earlier this year, investigated how an argon-helium process gas affected spatter formation and process stability during laser powder bed fusion (LPBF) printing of titanium lattice structures. The novel gas reportedly decreased spatter emissions by 35 percent and therefore has the potential to considerably reduce the risk of manufacturing defective parts.
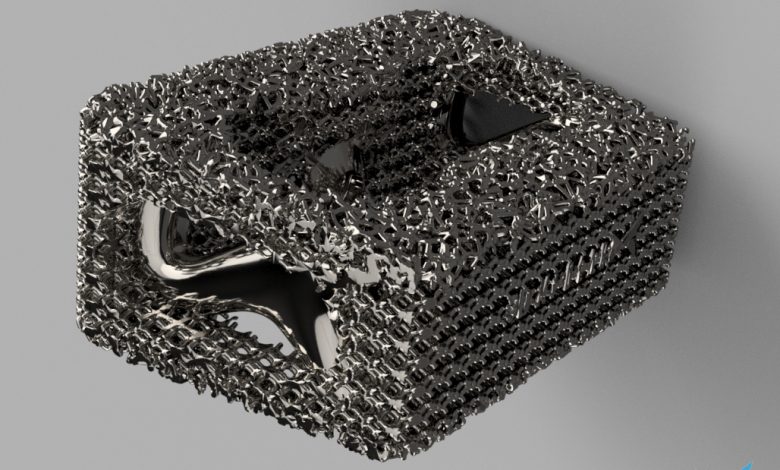
The study is part of a joint development program between the two companies to improve the quality and production of advanced thin medical devices made from the titanium alloy, Ti64.
“The ability to print reliability repeatable products is key to improving product qualification, which is crucial for the medical industry,” said Sophie Dubiez-Le Goff, Expert Powder Metallurgy for Additive Manufacturing at Linde. “Additionally, from a commercial perspective, printing time is the greatest single cost element in additive manufacturing, but this can be sped up for thin parts by using just the right atmospheric gas mixture.
“Linde’s novel argon-helium mix has been developed to do just that and is a major step forward in the manufacture of titanium medical devices.”
Linde and Medlab’s ongoing partnership
Linde and 3D Medlab first came together last year to research optimizing atmospheric conditions within the 3D printing process. The companies embarked upon a research project involving a series of atmospheric trials with the aim of developing a smoother and cleaner method of recycling gases during production.
The ultimate goal of the partnership is to create enhanced products for the medical sector, such as latticed orthopedic components that can better assimilate into a patient’s own bone and tissue structure.
Linde Group is known for its ADDvance O2 precision analysis technology, which aids metal 3D additive manufacturers to more accurately control the oxygen and humidity levels within a printer’s chamber. The company released an updated ADDvance powder cabinet for metal materials in 2018, designed to retain the quality of metal powders used during 3D printing.
Testing the argon-helium process gas
The LPBF 3D printing process facilitates the production of complex latticed structures, such as intricate medical implants, and utilizes a laser to melt consecutive layers of metal powder to form a solid part. The process is subject to a number of parameters that affect the melting behavior of the metal powder, which can in turn impact part quality and the speed of the printing process.
Porosity and surface quality are key factors in the mechanical properties of highly intricate 3D printed parts. From a medical device perspective, these elements can ensure the finished product is as close to the original design as possible while also reducing the amount of metal powder released into the human body from an implant.
Manufacturing on Demand
In order to improve these aspects within the LPBF printing process for thin medical devices, Linde and 3D Medlab conducted several atmospheric tests with their novel argon-helium process gas.
“Porosity is the first criteria we look at in terms of defining the quality of an additive manufactured medical device,” said Gael Volpi, Head of Additive Manufacturing at Marle Group, of which 3D Medlab is a part. “The results of our joint atmospheric gas study with Linde shows that the right balance of helium to argon in the process gas mixture – and ease of implementation – can make all the difference to both quality of output and productivity.”
Another critical element that can affect both overall part quality and printing speed is the inert gas within a machine’s print chamber. The partners’ study primarily sought to evaluate the ideal gas mixture that would optimize both of these outcomes.
First, they tested the effects of using argon alone within the print chamber during the printing of Ti64 parts. This led to a significant amount of spatter – molten metal particles caused by the laser – being splashed against adjacent parts being printed. Spatter on highly intricate parts can result in less fine quality of part threads and therefore reduce overall part quality. Using pure argon also resulted in porosity levels within the printed parts that could be “significantly improved” according to the engineers.
In light of this, the companies then tested Linde’s unique argon-helium gas mixture, using process monitoring and tomography images to identify the effects of the gas on the printed parts. The engineers observed spatter emission was considerably reduced when using the argon-helium gas compared to using argon alone.
The test results revealed that the argon-helium mixture decreased spatter emissions by 35 percent and in turn, reduced the risk of manufacturing defective parts while improving surface quality and part repeatability.
“Higher productivity was not reached at the expense of quality,” said Goff. “On the contrary, thanks to the new process gas mixture being so effective in reducing porosity content by 70 percent – according to microtomography analyses – compression properties remained comparable to parts processed with argon only.”
In addition to their current partnership focused on Ti64 lattice structures, the companies are reportedly planning to continue working together in the future to optimize the atmospheric printing conditions for materials such as nickel-titanium, which has great potential for manufacturing 3D printed surgical products such as next-generation stents.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Hayley Everett

Leave A Comment