A worldwide collective of researchers and scientists from universities, institutions, and hospitals have come together to produce a roadmap for 3D bioprinting.
The paper details the current state of bioprinting, including recent advances of the technology in selected applications as well as the present developments and challenges. It also envisions how the technology can improve in the future, and details the research that went into creating the roadmap.
Each of the authors takes on different aspects of bioprinting technology to focus on within the study. Specifically, these topics range from cell expansion and novel bioink development to cell/stem cell printing, from organoid-based tissue organization to bioprinting of human-scale tissue structures, and from building cell/tissue/organ-on-a-chip to biomanufacturing of multicellular engineered living systems.
Explaining the need for the bioprinting roadmap, the authors explain in the abstract of the paper that: “Due to the rapid pace of methodological advancements in bioprinting techniques and wide-ranging applications, the direction in which the field should advance is not immediately clear.”
“This bioprinting roadmap addresses this unmet need by providing a comprehensive summary and recommendations useful to experienced researchers and newcomers to the field.”
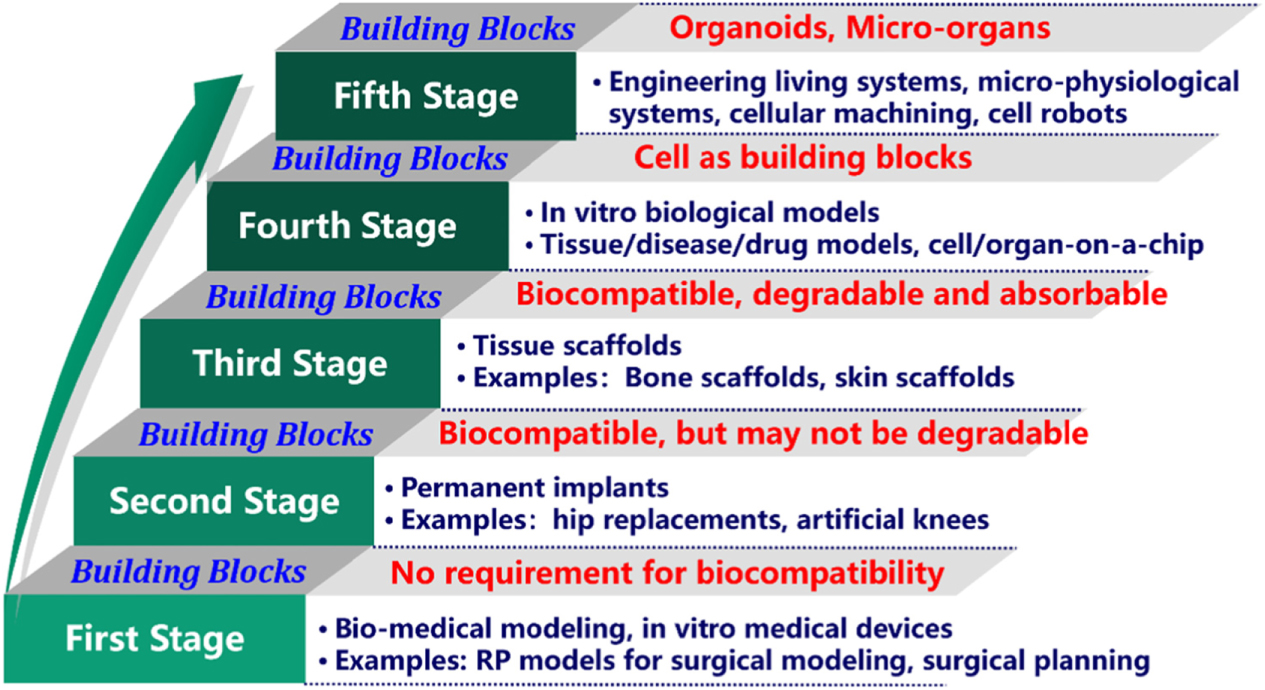
The bioprinting roadmap
In the introduction of the paper Wei Sun, chair professor of the College of Engineering from Drexel University, in Philadelphia, and Tsinghua University in Beijing, China, explains the challenges that must be overcome in bioprinting in general. These revolve around the creation of a new generation of bioinks better equipped for transporting, protecting, and growing cells; improved bioprinting processes; effective crosslinking; and integration with microfluidic devices to provide a long-term and a simulated physiological environment for culturing bio printed models.
The first section, “From cell expansion to 3D cell printing,” discusses the importance of cell expansion in the bioprinting process. It states that bioreactor-based cell-expansion systems need to be improved to increase the adoption of bioprinting in regenerative medicine and tissue engineering product markets. Bioreactor-based systems are able to expand cells much faster than traditional methods leveraging flat culture plate solutions.
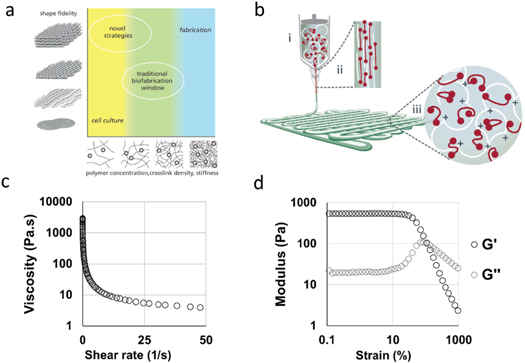
Moving on, the authors also take a look at the state of bioinks, critical to the bioprinting process. Despite significant progress in engineering bioinks for bioprinting, the paper states that further advances in the field are needed to facilitate the encapsulation of cells in microenvironments enabling improved replication of native tissues, organs, and their complexities.
Another topic covered is the bioprinting of stem cells. This is a field that maintains “great promise for biomedical research and applications, owing to their strong renewability as a cell source, and potential to differentiate and mature into many cell types in the human body,” according to the researchers. However, a number of hurdles and challenges remain to be overcome before the full potential of stem cell bioprinting can be realized. These hurdles are summarized into three areas: the physical printing effects stem cell bioprinting; the properties of the bioink; and the biological challenges of 3D cultured stem cells. Recently, researchers from the University of Toronto (UoT) and Sunnybrook Health Sciences Centre developed a handheld device capable of 3D bioprinting sheets of stem cells that can heal burn wounds.
Manufacturing on Demand
The next frontier for bioprinting
The paper goes on to discuss the “Large-scale and efficient production of organoids or cell aggregates.” Organoids are useful in that they effectively mimic the physiological microstructures of in vivo tissue or organs. A significant obstruction to the large-scale production of organoids is the high production costs and difficulty associated with producing them.
The researchers also explore 3D printed biohybrid tissues as in vitro biological models for studying diseases. They explain that 3D bioprinting holds the potential to create better disease models by enabling the rapid fabrication of tissues with accurate spatial arrangement of cells. A number of functions integral to in vivo tissue for the evaluation of drug responses are not successfully replicated in bioprinting. This includes multiple-layered barrier functions to control the transdermal delivery of external-use drugs, which the researchers believe can be replicated by creating 3D printed biohybrid tissues.
As well as further exploration of tissue assembly, organ-on-a-chip development, and multicellular engineered living systems, the paper also delves into bioprinting in outer space. Thanks to microgravity, one advantage of bioprinting in space is the creation of bioprinted constructs with more fluidic, biocompatible bionks. Additionally, microgravity conditions allow for the 3D bioprinting of tissue and organ constructs with more complex geometries, like voids, cavities, and tunnels.

Such an endeavor is already being explored by several research groups and companies in the USA, European Union, Russia, and China, who are actively preparing the necessary research infrastructure and devices for bioprinting in space. For example, the 3D BioFabrication Facility (BFF) bioprinter from nScrypt and Techshot is currently onboard International Space Station (ISS). This week, a payload containing 3D printed supplies for the BFF was delivered to the ISS, holding samples of human cells, bio-inks, and a set of new 3D printed ceramic fluid manifolds to replace the previously used printed polymers. 3D Bioprinting Solutions, a Russian bio-technical research laboratory also has its magnetic 3D bioprinter Organ.Aut onboard the ISS. The company was recently able to 3D bioprint bone tissue in zero gravity.
“The bioprinting roadmap” is published in , and is written by a number of authors, including Binil Starly, professor of mechanical engineering at North Carolina State University; Jason Burdick, a bioengineer from the University of Pennsylvania; Gregor Skeldon, a life science medical writer at Maverex, in the UK; Wenmiao Shu, professor of biomedical engineering at the University of Strathclyde in Glasgow; Andrew Daly, an orthopedic surgeon at Emory University Hospital; Jürgen Groll, professor of functional materials in medicine and dentistry at the University of Würzburg in Germany; Dong-woo Cho, mechanical engineer at Pohang University of Science and Technology; Vladimir A. Mironov, chief scientific officer of 3D Bioprinting Solutions.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Anas Essop


Leave A Comment