US 3D printer manufacturer 3D Systems has announced the formation of a new 3D bioprinting subsidiary.
Named Systemic Bio, the company is set to utilize the technologies developed as part of its parent firm’s Print to Perfusion program as well as those of its other subsidiary Allevi, to 3D bioprint vascularized organ models from human cells.
Backed with $15 million in seed funding from its owner, Systemic Bio now intends to market resulting tissues as a pharmaceutical drug discovery tool, with the aim of generating $100 million per year in revenue by 2027. These cells have already proven capable of accurately simulating human immune responses, and it’s thought they could help reduce drug R&D costs and lead times moving forwards.
“In forming Systemic Bio, we are applying these core technologies to specifically address critical needs within the pharmaceutical market,” said Dr. Jeffrey Graves, CEO of 3D Systems. “With our potential to ultimately manufacture hundreds or even thousands of custom-designed, proprietary human tissue models, pharmaceutical companies can more rapidly and accurately evaluate the efficacy of developmental drugs in the lab, with the goal of reducing development time.”
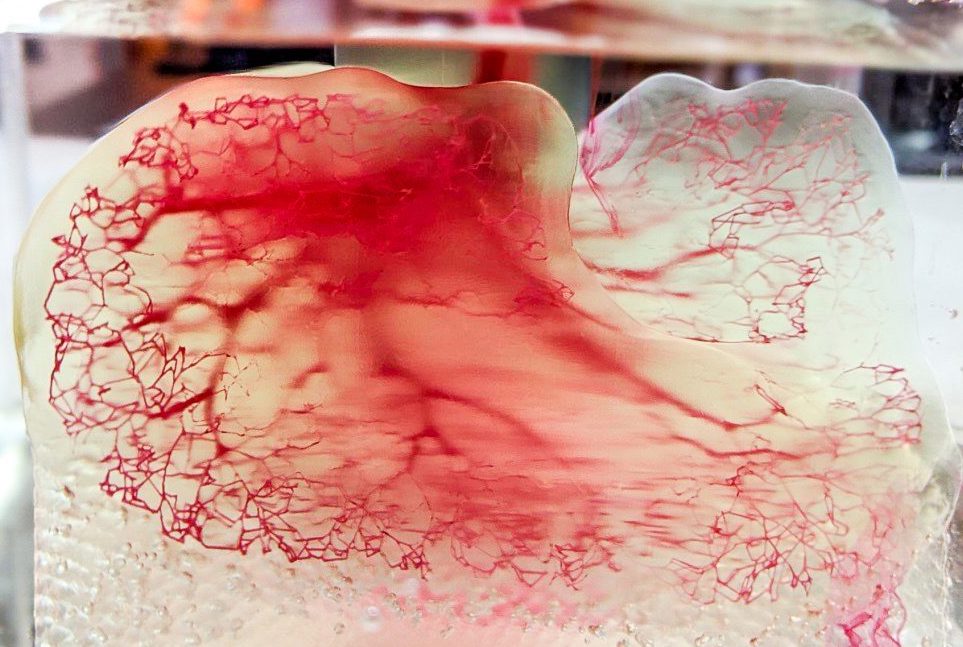
3D Systems’ 3D bioprinting efforts
Since mid-2020, 3D Systems’ strategic refocus has seen it prioritize its industrial and medical businesses, and invest heavily in 3D bioprinting R&D. Working with biotechnology specialist United Therapeutics, the firm has made significant advances in the development of Print to Perfusion, a process designed to enable the 3D printing of scaffolds that can be ‘perfused’ with living cells to create tissues.
Having announced a Print to Perfusion breakthrough in January 2021, in which it managed to create fully-sized solid-organ scaffolds, 3D Systems went on to scale its efforts by tailoring its Figure 4 3D printer to 3D bioprinting. The technology has previously been applied to 3D print custom hearing devices, and the Figure 4’s expanded material platform continues to see it find new 3D printing applications.
Later, in May 2021, 3D Systems acquired Allevi before taking over Volumetric Biotechnologies, in deals that could cost north of $400 million. Drawing on the experience accumulated via these moves, the firm has since made substantial R&D progress alongside United Therapeutics, to the point that they announced plans in June for 3D printed human lung scaffold trials within just five years.
“I am pleased and inspired by the progress we continue to make,” added Graves. “The complexity and precision that we have now demonstrated using biocompatible materials and our most advanced production bioprinting platform is truly groundbreaking, opening a host of new applications ranging from the laboratory to replacement organs within the human body.”
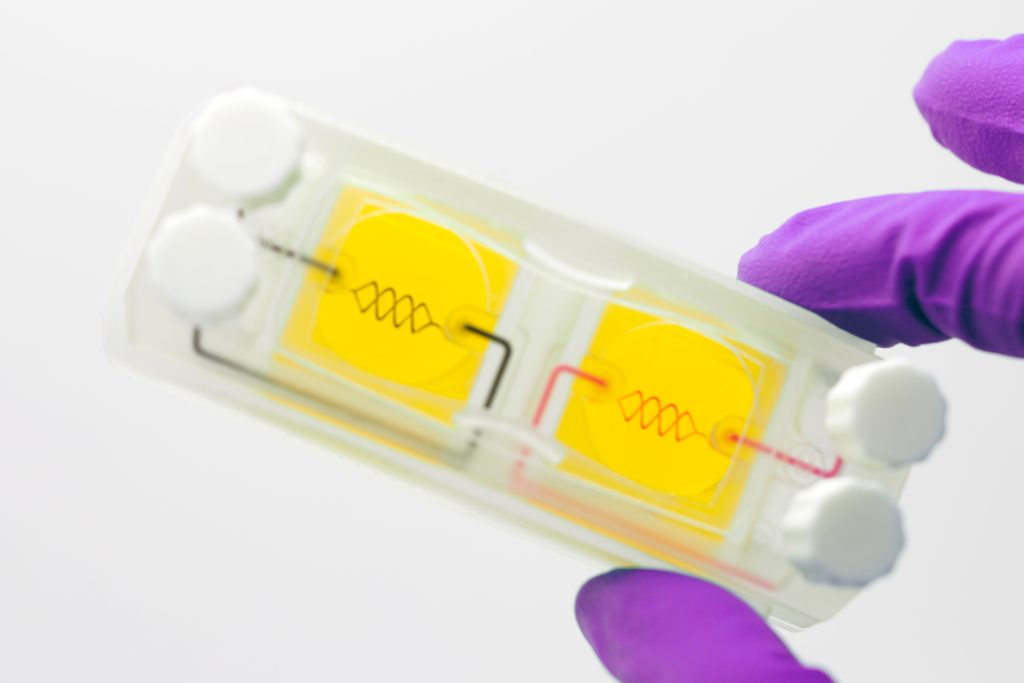
Systemic Bio’s ‘h-VIOS’ platform
3D Systems’ latest bioprinting expansion has seen it form a new fully-owned subsidiary that will focus primarily on the development and commercialization of its developed and acquired technologies. Using these, Systemic Bio plans to develop vascularized tissues for its proprietary human vascularized integrated organ system (h-VIOS) platform.
Manufacturing on Demand
Comprising plates of cellularized or acellular vascularized 3D scaffolds, and the accessories needed for drug testing, the organ-on-a-chip platform is said to boast several advantages over conventional technologies. Unlike traditional models which rely on synthetic materials, Systemic Bio’s deploys hydrogels which enable it to produce high-res tissues that more closely resemble their human counterparts.
Resulting scaffolds can also be seeded with both diseased and healthy human cells from different organs, unlocking the creation of tissues for drug safety and efficacy screening applications. At its facility in Houston, Texas, it’s said the custom chips can be produced at ten times the speed and resolution of current platforms to boot, which bodes well for the technology’s marketability and scalability.
Systemic Bio is said to be working to establish multi-phase partnerships with pharmaceutical companies that could soon lead to promising medicinal discoveries. At the helm of its new business, 3D Systems has appointed former Allevi CSO Taci Pereira, who is now spearheading its commercialization efforts, with the h-VIOS platform currently at the stage of being offered to select initial partners.
“As the leader of our Allevi business, Taci brought a unique blend of business acumen and bioprinting expertise that has enabled our continued growth,” said Menno Ellis, Executive VP of Healthcare Solutions at 3D Systems. “I’m confident that the solutions her team delivers can have a transformative impact in the field of pharmaceutical drug discovery and contribute meaningfully to the exciting growth we have envisioned for our healthcare business.”

Bioprinted organs edging closer to reality?
While 3D bioprinted organs are still many years away from being implant-ready, the technology behind them continues to make advances towards the development of tissues with drug discovery applications. Prellis Biologics has managed to develop a 3D bioprinted lymph node capable of recreating human immune responses, potentially making it an ideal disease therapy research tool.
Earlier this year, Trestle Biotherapeutics also licensed a process that combines stem cell and biofabrication technologies to enable the 3D bioprinting of functional kidney tissues. Using the process, the firm aims to create kidney scaffolds that could help get people off dialysis, and one day, it believes it may even be possible to deploy it in the production of entire organs.
Biolife4D, on the other hand, has filed for an IPO to raise the capital needed to commercialize the mini 3D bioprinted heart it has been developing for over four years. Through the move, the firm aims to bring in around $17.5 million, funding it expects to deploy in the expansion of its operations, although it admits its asking investors to back something that “has never been successfully done” before.
.
You might also like:
Ice 3D printing process could yield new biomedical and electronic devices with complex internal channels: This ‘inside-out’ 3D printing process involves jetting water droplets onto a custom-built platform, capable of freezing them upon landing at a temperature of -31 °F. These smooth, support-free ice sculptures can then be dunked in resin and cured, in a way that melts them, leaving behind parts with complex internal pathways.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment