US 3D printer manufacturer 3D Systems has announced its entry into the radiation oncology market with the launch of its new Virtual Surgical Planning or ‘VSP’ Bolus solution.
Designed to enable the production of medical devices for cancer treatments, the VSP Bolus service allows clinicians to 3D print them at tailored thicknesses, reducing any air gaps between instruments and patients. This snug level of fit not only makes resulting parts created from a biocompatible elastomer more comfortable, but potentially improves their therapeutic efficacy and patients’ survival chances.
“While radiotherapy has become recognized as a common course of treatment for cancer diagnoses, each case is as unique as each patient,” explains Menno Ellis, Executive VP of Healthcare Solutions at 3D Systems. “Blending the experience of our biomedical engineers, our biocompatible materials, 3D printing technology, and best-in-class digital workflows, we are able to both design and produce patient-specific devices to help improve the delivery of radiotherapy treatment.”
“Our new VSP Bolus product expands our capabilities to address yet another incredibly important application for personalized healthcare.”
3D Systems’ VSP portfolio
With a broad 3D printer, material and software portfolio, 3D Systems is currently able to address the manufacturing needs of clients operating in a wide variety of industrial and medical verticals. As one of the core pillars of its offering in the latter, the firm’s VSP Surgical Planning services currently see it work with clinical partners, to develop custom medical solutions that improve patient outcomes.
Carried out using 3D Systems’ suite of 3D printing resources, these services meet a host of healthcare needs, from virtual surgical planning right through to anatomic modeling and the creation of craniomaxillofacial and orthopaedic devices.
In order to facilitate such projects, the firm continues to seek FDA approval, both for its services and using new materials within medical applications. Since gaining 510(k) clearance for its VSP Orthopaedics platform, 3D Systems has gone on to receive the same FDA approval for using LaserForm Ti and DuraForm ProX– to create maxillofacial guides.
The company also added new VSP Hybrid Maxillofacial Guides to its VSP Surgical Planning portfolio in April 2021. Designed to help surgeons operate with greater precision and confidence, the devices combine the strength of titanium with nylon’s softness, making them robust yet malleable enough for occlusal registration.
Moving into radiation oncology
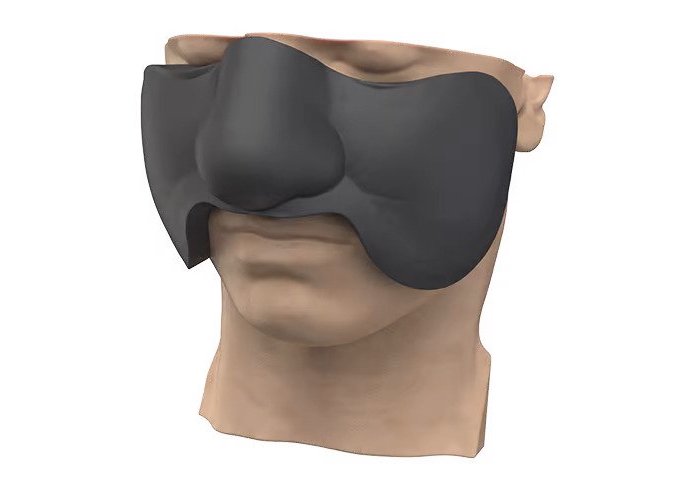
According to data cited by 3D Systems itself, around half of all those diagnosed with cancer will, at some point in their treatment, undergo radiotherapy. During the procedure, patients are required to wear something called a ‘bolus,’ a flexible device designed to conform tightly to their skin, and help target radiation wherever in the body it’s needed.
Manufacturing on Demand
However, 3D Systems says many current devices leave spaces between the bolus and the patient’s anatomy, which can result in cancerous cells not getting the required dose, or even leave adjacent areas of the body vulnerable to unneeded radiation. Producing such energy modulating devices repeatedly is also said to be difficult for clinical staff, especially as they balance trying to do so with their duties.
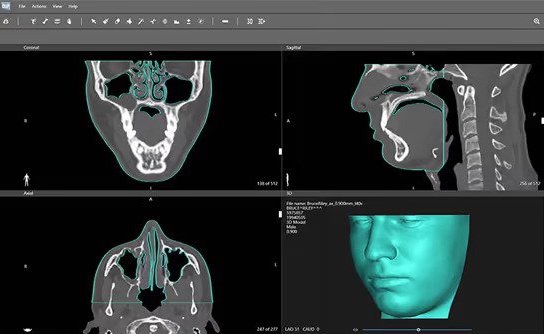
To help medical physicists, dosimetrists and radiation oncologists overcome these issues, 3D Systems has therefore developed an alternative 3D printing workflow, which it calls VSP Bolus. In the company’s revised setup, clinicians are able to submit a patient’s CT scan data, which can then in turn, be segmented and used as a basis for a custom bolus designed via its DICOM To Print (D2P) software.
Once ready, the device is sent for production at one of 3D Systems’ certified medical instrument manufacturing facilities, in a way that frees radiotherapy staff from having to create it from scratch themselves.
As well as recently gaining FDA 510(k) clearance, the company says its service also eliminates the need for any barriers between devices and patients’ skin, while enabling the production of reusable boluses. This, in the words of 3D Systems, is unique, as it makes VSP Bolus the “only solution on the market that offers a full design and production service based on a patient’s treatment plan.”
3D printing in radiotherapy
Though they may not quite have the resources required to roll them out at the same scale as 3D Systems does, there are several other companies and research groups also investigating the potential of 3D printed radiotherapy devices. Back in 2020, Adaptiiv Medical Technologies raised $1.8 million towards the development of its oncological device 3D modeling software.
At North Carolina State University, on the other hand, researchers have used 3D printing to develop more comfortable radiation-receiving antennas for cancer patients to wear during microwave breast hyperthermia procedures. By tweaking their device’s level of infill, the team behind the project has even been able to adjust its dielectric constant, optimizing its performance at different exposure levels.
A collaborative group based at the Dana-Farber Cancer Institute, Brigham and Women’s Hospital, Massachusetts General Hospital and MIT have also utilized the technology to create gastro-radiation shielding for cancer patients. Using the custom devices, which prevent gamma and X-ray-induced radiation toxicity in tissues, it’s predicted that unneeded exposure could be reduced by up to 30%.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Paul Hanaphy

Leave A Comment