Chinese drug 3D printing firm Triastek has concluded its First-in-Human (FIH) study of its new 3D printing drug, dubbed T21. This drug is designed to treat moderate to severe ulcerative colitis (UC).
According to the study’s imaging findings, T21 tablets demonstrate accurate delivery and controlled release to the colon, where the drug is intended to take effect. The tablets are produced using the company’s Melt Extrusion Deposition (MED) 3D printing process, which allows for precise regulation of the drug’s release in the gastrointestinal tract. As a result, this technology ensures a more focused and effective drug delivery mechanism.
“The first human study data with T21 verifies the precise colon delivery capability of the MED process, and this platform is poised to become the novel drug delivery system of choice for new colon-targeted products with either local efficacy or systemic absorption,” said Professor Xiaoling Li, Co-Founder and Chief Scientific Officer of Triastek. “We hope to continue showcasing how Triastek’s 3D printing processes can bring technical solutions to pharmaceutical companies for efficient product development of optimized drug delivery, ultimately leading to the ability to provide patients with more clinically valuable medicines.”
 Triastek receives FDA IND clearance for its T21 drug. Image via Triastek.
Triastek receives FDA IND clearance for its T21 drug. Image via Triastek.
Targeted and effective drug delivery
According to the company, oral medication is preferred for UC patients due to safety, pain avoidance, and patient compliance. Triastek’s T21 enables precise and targeted drug delivery to the gastrointestinal tract, improving effectiveness.
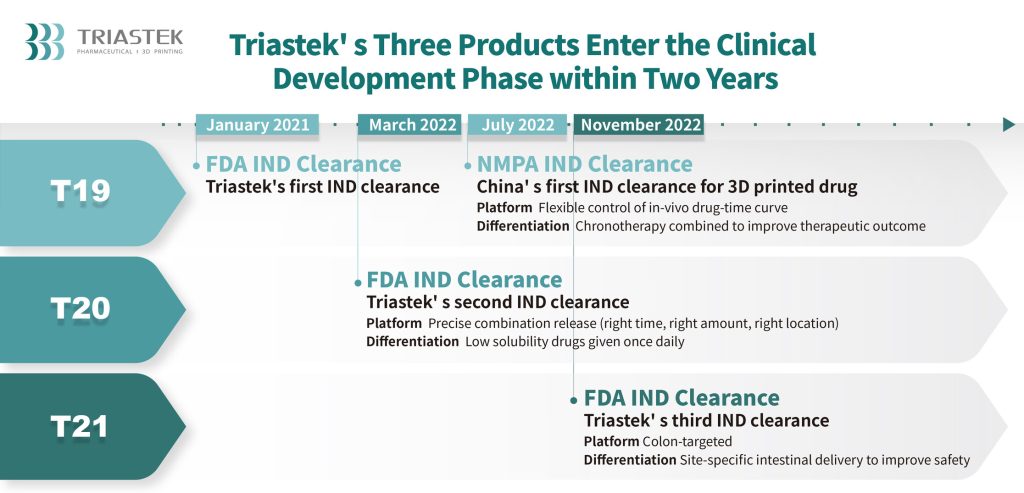
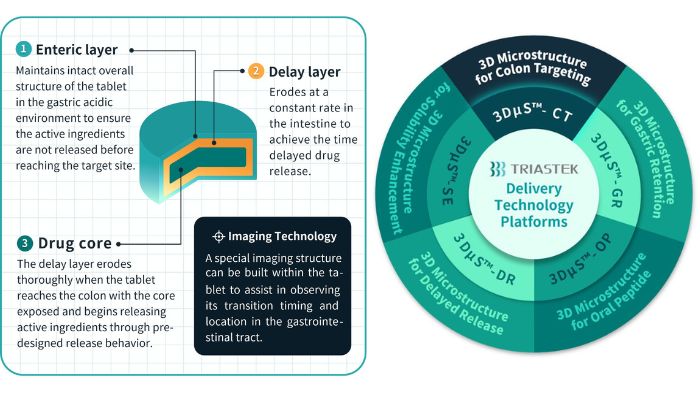
T21 is the company’s third 3D printed drug product and serves as an oral drug comprising a total of 3 layers namely, the enteric layer, delay layer, and drug core. The enteric layer maintains the tablet’s structure in the gastric acidic environment, ensuring active ingredients are not released prematurely. The delay layer erodes at a constant rate in the intestine, achieving time-delayed drug release. Upon reaching the colon, the delay layer fully erodes, releasing active ingredients in a targeted manner.
T21 obtained clearance for its Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) under the 505(B)(2) pathway, on November 18, 2022. Derived from the oral Janus kinase (JAK) inhibitor called tofacitinib, T21 was approved, leading Triastek to initiate FIH research in Q1 of 2023. The FIH study also aimed to examine the transport, erosion process, and release site of T21 in the human body following oral administration.
Triastek’s objective is to establish collaborations with pharmaceutical companies to leverage the advantages of 3D printing in medication production. One of its recent partnerships involves Boehringer Ingelheim, Eli Lilly, and Merck KGaA.
The total addressable market for 3D printed drugs
According to Triastek, drug 3D printing technology can be widely used for chemical drugs, biological drugs and peptides. Triastek’s MED technology offers end-to-end solutions, including novel dosage form design (multi-layered, honeycomb/weave and multi-compartments, etc.), FbD product development, and continuous manufacturing at commercial scale. The company’s 3D printing formulation platform, 3DFbD, translates target Pharmacokinetic profile (PK) profiles into corresponding release profiles. It enables precise tablet design for specific, hard-to-reach GI regions like the colon, ensuring controlled API release at the desired onset time, rate, and amount.
MED technology also aids in formulating challenge compounds to overcome biopharmaceutical deficiencies via gastro-retention and enhancing oral absorption of biologics. Triastek offers a commercial-scale MED 3D printing system capable of producing up to 50M tablets yearly. It accommodates both “blockbuster and rare disease products,” enabling flexible manufacturing.
Manufacturing on Demand
Furthermore, Triastek notes that the pharmaceutical preparation market is divided into solid and non-solid areas. 3D printing can be applied to produce solid preparations, which are dominated by small-molecule drugs. As per a report from Evaluate Pharma referenced by Triastek, the worldwide drug market was $894 Billion in 2022 and is expected to reach $1,236 Billion in 2028. Additionally, The worldwide small molecule drug market was $433 Billion in 2022 and is expected to reach $549 Billion in 2028.
Professor Xiaoling Li gave an indication of how much of the global pharmaceutical market might be addressable by 3D printing. “3D printing for pharmaceuticals is in its infancy. Current applications of 3D printing for pharmaceuticals have been mostly for small molecule drugs, with some exploratory studies for peptides and other biomolecules in the oral delivery areas via personalized dosing or mass production. Other areas of applications include API synthesis, implants, and drug device combinations. It is premature to estimate the share of 3D printed pharmaceuticals in the projected sales. However, the share of 3D printed pharmaceuticals will steadily increase as this technology is taking a foothold in above mentioned pharmaceutical areas,” added the Co-Founder and CSO.
 Layers of the T21 drug and Triastek’s other delivery technology platforms. Image via Triastek.
Layers of the T21 drug and Triastek’s other delivery technology platforms. Image via Triastek.
3D printing technology revolutionizes drug production
Last year, global pharmaceuticals company Eli Lilly and Triastek joined forces to research and develop 3D printed oral drugs for the gastrointestinal tract. The project leveraged Triastek’s proprietary MED technology to fabricate programmed drug release profiles, targeting specific areas of the human digestive system. Dr. Senping Cheng, the Founder, and CEO of Triastek, expressed that the collaboration with Lilly demonstrates the application of MED technology to enhance drug delivery via the oral route. The company envisions that the utilization of MED technology can address formulation challenges and lead to the development of clinically significant products for its worldwide partners.
Researchers from the MERLN Institute, University of Santiago de Compostela, University College London (UCL), and the UCL spin-out FabRx devised a way to 3D print tablets in just seven seconds. Unlike the traditional vat photopolymerization process used for layer-by-layer printing of pills, the team employed a volumetric 3D printing approach that cured full vats of resin in a single run. This advancement had the potential to accelerate the production rate of customized medication, proving to be a critical factor in making end-use clinical 3D printing more feasible.
You might also like:
Lithoz and Himed announce partnership to accelerate 3D printed medical bioceramic development: The partnership will focus on the development of medical implants using biocompatible calcium phosphates (CaP), seeking to meet growing demand for 3D printable bioceramic feedstocks.
* This article is reprinted from 3D Printing Industry. If you are involved in infringement, please contact us to delete it.
Author: Ada Shaikhnag

Leave A Comment