Nivalon Leverages AI and 3D Printing to Create Personalized, Motion-Preserving Spinal Implants
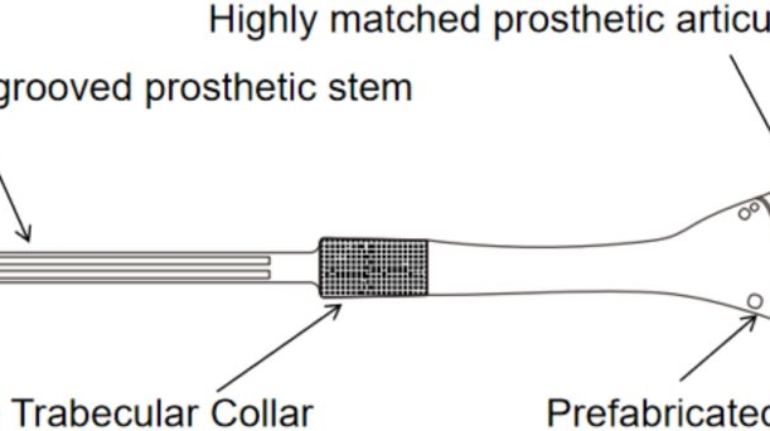
U.S. company creating patient-specific spinal implants Nivalon Medical Technologies has developed what it describes as the first fully patient-specific spinal implant that preserves natural motion without metal components. The device combines AI-driven design with advanced ceramic 3D printing, using a zirconia-toughened alumina (ZTA) structure and a flexible core to replicate spinal movement.