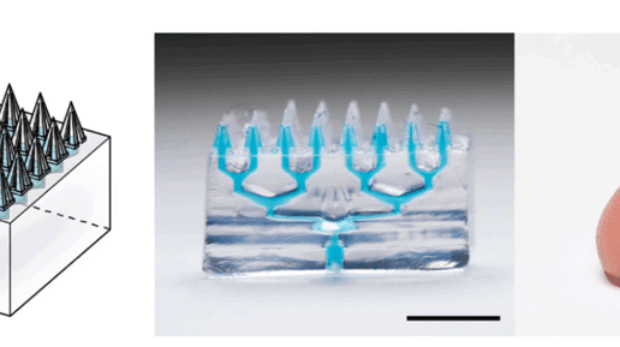
UCLA’s affordable AI-powered magnetic pen for early Parkinson’s diagnosis
Researchers at the University of California, Los Angeles (UCLA) have created a 3D printed pen that detects subtle tremors in handwriting, offering a potential tool for diagnosing Parkinson’s disease.