Lung Operation on Young Girl in Israel Aided by 3D Printing
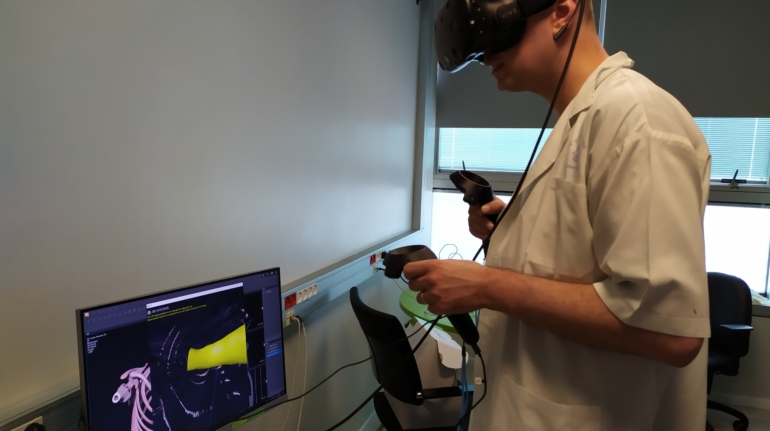
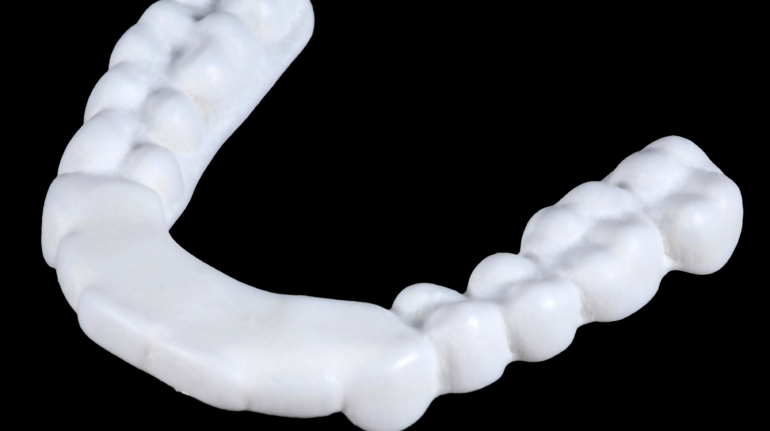
An anaesthesia team in Israel recently used 3D printing and virtual reality to produce an exact model of the airway of a 7-year-old girl, as part of an operation to remove a section of her lung.