German pharmaceutical giant Bayer has signed a cooperation agreement to test new drugs on human heart tissue 3D printed by researchers at Tel Aviv University.
Ramot, the university’s technology transfer department, said on Sunday that researchers working in the laboratory of Professor Tal Dvir will work with Bayer to use 3D printed heart tissue and ultimately the entire human body 3D printed hearts test the toxicity and efficacy of new drugs over the years.
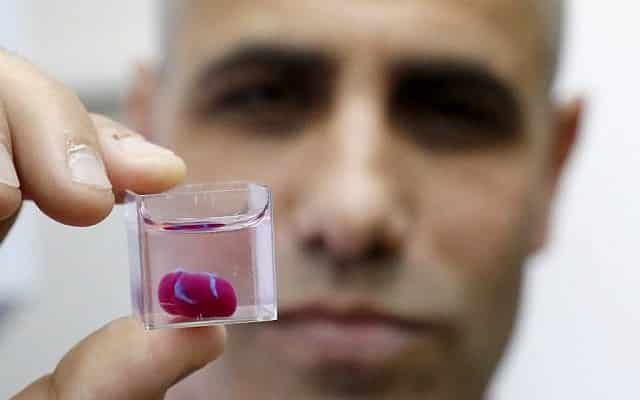
In April last year, Dvir’s laboratory unveiled the world’s first 3D printed heart with human tissue and blood vessels, calling it a major medical breakthrough.
Lamot announced in a statement on Sunday that the collaboration with Bayer will allow researchers to estimate that personalized organs and tissues can be printed within 10 to 15 years, thereby eliminating the need for organ donation and the risk of transplant rejection.
Lamot said in a statement that before this, this innovative technology had “revolutionary” potential and could change another medical field in the field of drug screening.
Candidate drugs go through several screening stages before reaching the pharmacy. First, test new compounds in human tissue cultures on petri dishes in the laboratory. Then, it was applied to experimental animals. Finally, the drug was approved for human clinical trials. The statement said that Dvir’s 3D printed cotton paper can achieve faster, cheaper and more efficient screening than petri dishes in the laboratory.
“In a Petri dish, all the cells line up in 2D, and it’s only one type of cell,” said Dvir in the statement. “In contrast, our engineered tissues are 3D-printed, and therefore better resembles real heart tissues. Our printed tissues contain cardiac muscle, blood vessels and the extracellular matrix which connects the different cells biochemically, mechanically and electrically. Moving away from Petri dishes to 3D-printed tissues could significantly improve drug tests, saving precious time and money with the hope of producing safer and more effective medication.”
Deville said he hoped that the cooperation with Bayer will allow preclinical trials on complete printed organs in the near future.
“Our agreement is just the beginning,” said Dvir. “Our end goal is to engineer whole human hearts, including all the different chambers, valves, arteries and veins,” for an “even better toxicological screening process.”

Dvir and co-founder Alon Sinai have obtained a technical license from Ramot and established a spin-off company called Matricelf, which focuses on creating personalized spinal cord implants to treat paralyzed patients.
According to data from the database Start-Up Nation Finder, Matricelf received an investment of $1 million in May. Ramot stated that the funding will enable the startup to reach a clinical environment in the near future.
“This collaboration with Bayer will support the evaluation and development of new drugs and is a step in building long-term relations with Bayer that we hope will benefit both partners and ultimately patients,” said Keren Primor Cohen, the CEO of Ramot, in the statement.
Eckhard von Keutz, head of transformation science at Bayer, said in a statement that the new collaboration with Tel Aviv University “will address new areas of early assessment of drug candidate safety and tolerability.” “We already have a global network of partners. This new project will allow Bayer to expand its open innovation activities to Israel, thereby providing a dynamic ecosystem for innovation in biotechnology and medical research.”