Leading software and 3D printing service provider Materialise has contributed €2.5 million to a €4 million funding round in Fluidda. Headquartered in Kontich, Belgium, Fluidda is a biotechnology company specializing in personalized imaging solutions for respiratory diseases, i.e., pulmonology.
As a result of the investment Wilfried Vancraen, founder and CEO of Materialise with 30 years of experience in 3D printing in the medical field, will join the board of directors at Fluidda.
“Fluidda was one of the first users of the pulmonology features of the and it is a nice example of how new technologies for healthcare diagnostics are being developed in collaboration with Materialise’s expertise in medical engineering,” said Vancraen.
“With this partnership we can help even more patients, bringing the possibilities of 3D printing and planning to the pulmonology market.”
Functional Respiratory Imaging
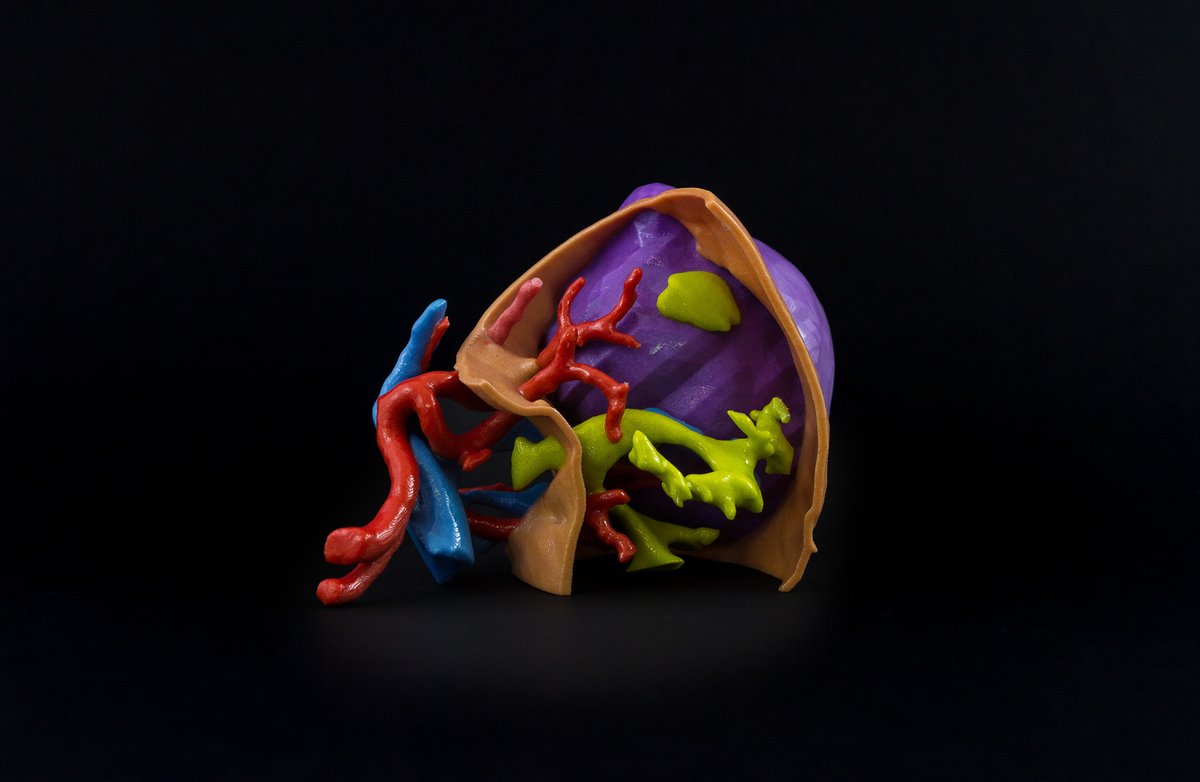
Founded in 2005, Fluidda’s main goal is to develop imaging biomarkers to improve drug development and patient care. Its proprietary imaging technology, Functional Respiratory Imaging (FRI), is based on airflow monitoring and CT scans and provides enhanced visualizations of the lung airflow in patients.
FRI aims to better equip medical professionals in treating those ailing from progressive lung diseases such as Chronic Obstructive Pulmonary Disease (COPD) and asthma. According to Fluidda, such conditions have been seen to increase over the past few years.
The partnership between Fluidda and Materialise will look to develop medical 3D printing and planning solutions, including 3D printed surgical models of lungs and its vascular structures, to increase the quality of care for these patients. Jan De Backer, CEO of Fluidda, added:
“We share the vision that the medical field, and particularly respiratory healthcare imaging needs to evolve towards personalized, precision medicine through a value-based healthcare approach. We are confident that advanced imaging techniques, such as our Functional Respiratory Imaging methods, can add value in this regard.”
Materialise and the medical sector
Though Materialise provides 3D printing solutions to a variety of industries it has particular strength in medical. Through its manufacturing service, the compnay produces 3D printed implants, as well as pre-surgical guides for planning, and anatomical models.
In 2018 the Materialise Mimics software package was granted ’ 510(k) clearance by the U.S. Food and Drug Administration (FDA), a classification which was recently renewed for 2019. The company is also now providing validation for 3D printers to go into hospitals.
Vote for your Medical, Dental or Healthcare Application of the Year in the 2019 Industry Awards.
Subscribe to our newsletter and follow us Facebook and Twitter for the latest additive manufacturing updates. Visit our Jobs board to find out more about opportunities in additive manufacturing.
Featured image shows a model representing a respiratory disease in a patient’s lung. Photo via Fluidda.

Leave A Comment