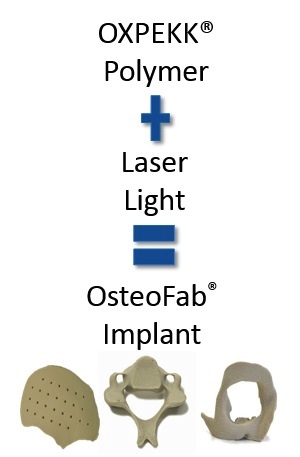
One of the world’s most prestigious surgical implant companies are turning to some very special 3D printing technology to help give their medical products a stronger backbone. Florida-based RTI Surgical has been granted an exclusive license to the medically driven 3D printing technology created by Oxford Performance Materials. The agreement will allow RTI Surgical to utilize OPM’s OsteoFab technology platform for spinal implant parts, which are manufactured from their biocompatible OXPEKK polymer on an EOSINT P800 industrial SLS 3D printing system. The OXPEKK polymer, which is known for its strong and pure material characteristics, is already successfully proven in orthopedic implants, and will certainly stand to help assist spinal surgeons across the globe.
“We are thrilled to announce this agreement with Oxford Performance Materials,” said Brian K. Hutchison, the CEO of RTI Surgical. “OsteoFab is an incredibly exciting technology that creates new opportunities for innovation in the spinal implant market. We look forward to working with OPM to offer our customers and their patients a new alternative for spinal implants.”
What makes OPM’s 3D printing technology so valuable to RTI Surgical, and likely to many others across the medical industry, is that the OsteoFab process and OXPEKK polymer creates a bone implant that actually enables bone on-growth, is able to be x-rayed, and possesses bone-like characteristics. Though OPM will play a huge role in the production of these 3D printed spinal implants, RTI Surgical will still take care of the finishing steps, packaging, marketing, and distribution of the products. Outside of spinal implants, OPM has also succeeded in 3D printing biocompatible facial implants with their OsteoFab technology. After seeing the wide and innovative reach of RTI Surgical, OPM decided to strike up a deal to license and spread their groundbreaking technology further throughout the medical field.
“We see RTI Surgical as a growth company in the spinal implant space,” said Scott DeFelice, the CEO of OPM. “RTI is a leading developer of medical implants with a long history of putting sound science behind their products. From the outset, RTI has worked closely with us and we look forward to a lengthy and productive relationship that leverages the unique benefits of OsteoFab technology to improve patient care.”
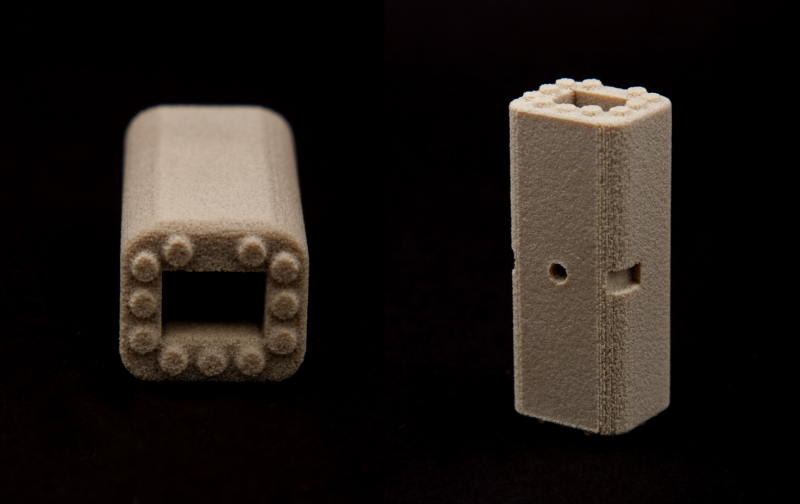
The agreement is sure to enhance RTI Surgical’s access to viable spinal implants, and thus the new alternative will likely help many in need of spinal surgery. By offering their biocompatible 3D printed bone-like material to RTI Surgical, OPM is showing that when it comes to the intensive surgery involving skeletal implants, they’ve (literally) got our backs.

Leave A Comment