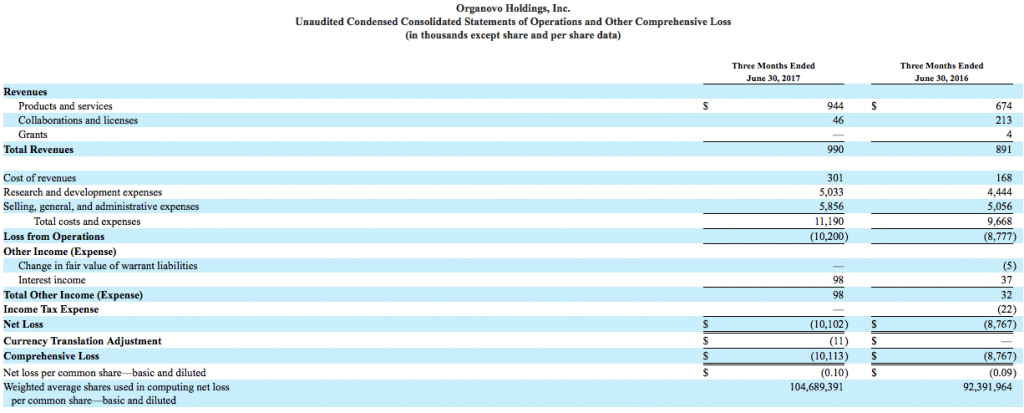
Following a demand for tissue development research, 3D bioprinting material provider Organovo (NASDAQ:ONVO) has reported a total revenue of $1.0 million for the first quarter of FY 2018, an 11% increase on the same period in 2017.
With the results, the company also affirms its outlook for the full-year, expecting total revenue to reach $6.0 million – $8.5 million by the end of FY 2018, compared to annual turnover of $4.2 million in 2017.

Operations and services
Total loss from operations in Q1 2018 was $10,200,000, and Q1 2017 reported $8,777,000. The increased expenditure was received across the board in cost of revenues, general and administrative expenses, and research and development.
In Q1 2017, Organovo revenue from products and services was valued at $674,000 for period ending June 30 2016. In the same period of Q1 2018, revenue gathered from tissue sales and research as a service created an increase to $944,000.
In a statement on the report Organovo CEO Taylor J. Crouch comments, “Demand for compound screening in disease models is growing, with new applications frequently emerging as clients seek novel solutions in their drug discovery workflow.”
Meeting demand
Placing special emphasis on the company’s R&D activities, Crouch highlights requests for new biological models, and the success of tissue treatments in animals. He asserts that around 70% of tissue research service requests were received from existing clients.
In this period, the company also announced a collaboration with the University of Virginia to develop treatment for volumetric muscle loss (VML).
New organ model requests
New requests from Organovo clients have been to create true-to-life cells models of Hepatitis B and ribonucleic acid (RNA) which is involved in the transmission of genetic data.
To be 3D bioprinted in lab-on-a-chip type devices, the cell models may be used to test drugs and other substances, or to develop therapeutic patches for parts of the body.
Throughout FY 2018, Organovo is committed to continuing live studies of its proprietary liver and kidney materials. Crouch also confirmed that the company will start seeking orphan designation of its tissues.
U.S. designation would mean that Organovo products could be used treat a rare disease or condition upon request.
Closing statements
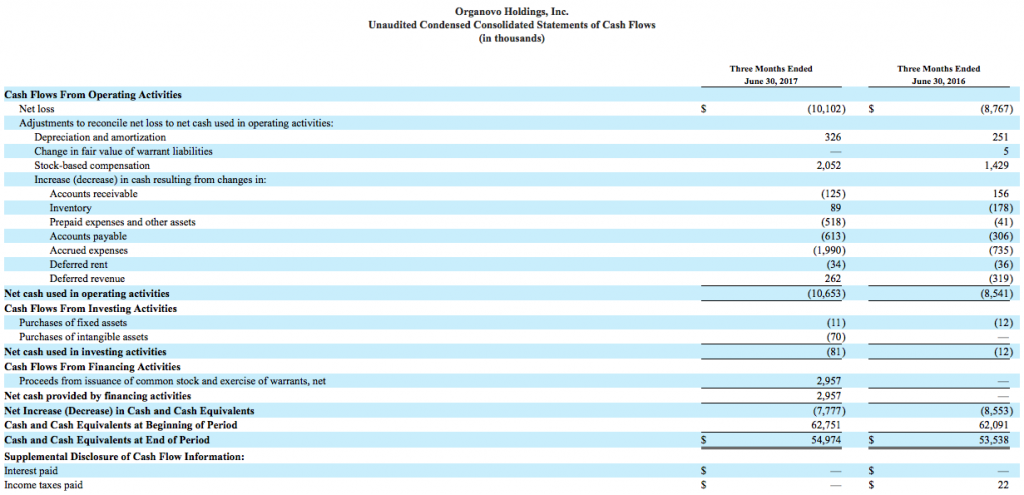
Organovo’s consolidated statement of operations and balance sheet can be viewed below.
Concluding the release Crouch comments, “Our long-term plan remains founded on targeting attractive and growing markets with critical unmet needs, extending our first mover advantage, and capitalizing on our technology leadership to grow our product and service offerings.”
A full report of the Organovo’s earnings in Q1 2018 can be viewed online here.
To stay up to date with the latest financial reports and other news sign up to the Industry newsletter, follow us on Twitter and like us on Facebook.
Make your move in the industry and register on our 3D printing jobs site.
Featured image: Bio-fluorescence in ExVive 3D printed human liver tissue. Image via Organovo

Leave A Comment